Invega Trinza Prescribing Information
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. INVEGA TRINZA is not approved for the treatment of patients with dementia-related psychosis
Dosage and Administration (INVEGA TRINZA has not been systematically studied in patients with renal impairment [see Clinical Pharmacology (12.3)] . For patients with mild renal impairment (creatinine clearance ≥ 50 mL/min to < 80 mL/min [Cockcroft-Gault Formula]), adjust dosage and stabilize the patient using the 1-month paliperidone palmitate extended-release injectable suspension, then transition to INVEGA TRINZA(see Table 1) [see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)]. Refer to the Prescribing Information of the 1-month paliperidone palmitate extended-release injectable suspension product for the recommended dosage in patients with mild renal impairment.INVEGA TRINZA is not recommended in patients with moderate or severe renal impairment (creatinine clearance < 50 mL/min) [see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)] .
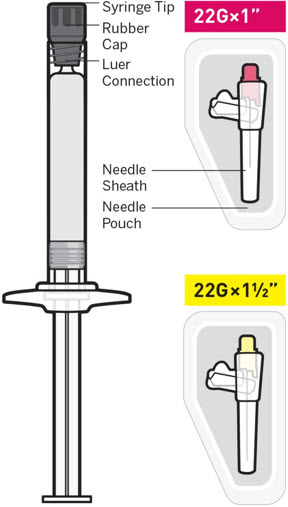
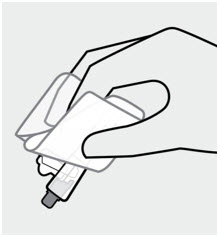
For intramuscular injection only. Do not administer by any other route.Important INVEGA TRINZA should be administered by a healthcare professional as a single injection. DO NOT divide dose into multiple injections.INVEGA TRINZA is intended for intramuscular use only. Inject slowly, deep into the muscle taking care to avoid injection into a blood vessel. Read complete instructions prior to use. Dosing This medication should be administered once every 3 months .Preparation Peel off tab label from the syringe and place in patient record. INVEGA TRINZA requires longer and more vigorous shaking than INVEGA SUSTENNA (1-month paliperidone palmitate extended-release injectable suspension). Shake the syringe vigorously, with the syringe tip pointing up, for at least 15 seconds within 5 minutes prior to administration (see Step 2).Thin Wall Safety Needle Selection Thin wall safety needles are designed to be used with INVEGA TRINZA. Therefore, it is important to only use the needles provided in the INVEGA TRINZA kit .Dose pack contents
1 Select needle Needle selection is determined by injection area and patient weight.
2 Prepare for injection
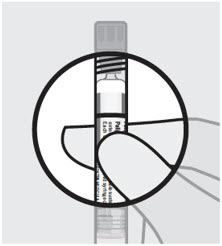
Check suspension
After shaking the syringe for at least 15 seconds, check the liquid in the viewing window. The suspension should appear uniform and milky white in color. It is also normal to see small air bubbles. Open needle pouch and remove cap
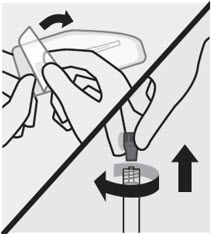
First, open needle pouch by peeling the cover back half way. Place on a clean surface. Then, holding the syringe upright, twist and pull the rubber cap to remove. Grasp needle pouch
Fold back needle cover and plastic tray. Then, firmly grasp the needle sheath through the pouch, as shown. Attach needle
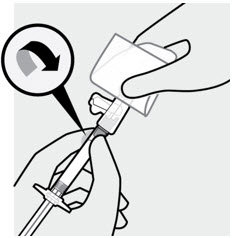
Hold the syringe pointing up. Attach the safety needle to the syringe using a gentle twisting motion to avoid needle hub cracks or damage. Always check for signs of damage or leakage prior to administration. Remove needle sheath
Pull the needle sheath away from the needle in a straight motion. Do not twist the sheath, as this may loosen the needle from the syringe.Remove air bubbles
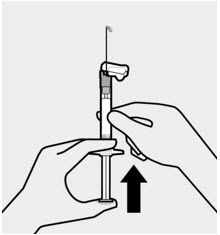
Hold the syringe upright and tap gently to make any air bubbles rise to the top. Remove air by pressing the plunger rod upward carefully until a drop of liquid comes out of the needle tip. 3 Inject Inject dose
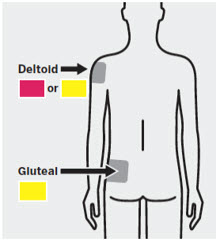
Slowly inject the entire contents of the syringe intramuscularly, deep into the selected deltoid or gluteal muscle.Do not administer by any other route. 4 After injection Secure needle
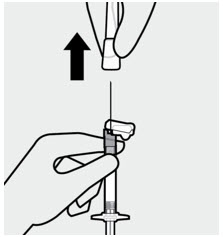
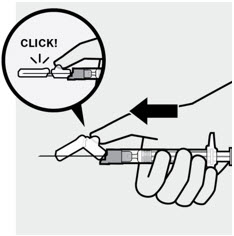
After the injection is complete, use your thumb or a flat surface to secure the needle in the safety device. The needle is secure when a "click" sound is heard. Dispose properly
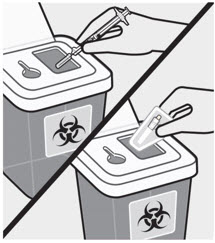
Dispose of the syringe and unused needle in an approved sharps container.
                     | 9/2024 | |||||||||||||||||||||||
Warnings and Precautions (Like other drugs that antagonize dopamine D2receptors, paliperidone elevates prolactin levels and the elevation persists during chronic administration. Paliperidone has a prolactin-elevating effect similar to that seen with risperidone, a drug that is associated with higher levels of prolactin than other antipsychotic drugs. Hyperprolactinemia, regardless of etiology, may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotrophin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro , a factor of potential importance if the prescription of these drugs is considered in a patient with previously detected breast cancer. An increase in the incidence of pituitary gland, mammary gland, and pancreatic islet cell neoplasia (mammary adenocarcinomas, pituitary and pancreatic adenomas) was observed in the risperidone carcinogenicity studies conducted in mice and rats[see Nonclinical Toxicology (13.1)] . Published epidemiologic studies have shown inconsistent results when exploring the potential association between hyperprolactinemia and breast cancer.In a long-term maintenance trial of INVEGA TRINZA, elevations of prolactin to above the reference range (>13.13 ng/mL in males and >26.72 ng/mL in females) relative to open-label baseline at any time during the double-blind phase were noted in a higher percentage of males in the INVEGA TRINZA group than in the placebo group (46% vs. 25%) and in a higher percentage of females in the INVEGA TRINZA group than in the placebo group (32% vs. 15%). During the double-blind phase, 1 female (2.4%) in the INVEGA TRINZA group experienced an adverse reaction of amenorrhea, while no potentially prolactin-related adverse reactions were noted among females in the placebo group. There were no potentially prolactin-related adverse reactions among males in either group. Prior to the double-blind phase (during the 29-week open-label phase of the long-term maintenance trial), the mean (SD) serum prolactin values at baseline in males (N=368) were 17.1 (13.55) ng/mL and 51.6 (40.85) ng/mL in females (N=122). Twelve weeks after a single injection of INVEGA TRINZA at the end of the open-label phase, mean (SD) prolactin values were 25.8 (13.49) ng/mL in males (N=322) and 70.6 (40.23) ng/mL in females (N=107). During the open-label phases 27% of females and 42% of males experienced elevations of prolactin above the reference range relative to baseline, and a higher proportion of females experienced potentially prolactin-related adverse reactions compared to males (7.9% vs. 3.7%). Amenorrhea (4.7%) and galactorrhea (3.1%) were the most commonly observed (≥3%) potentially prolactin-related adverse reactions in females. Among males in the open-label phase, no potentially prolactin-related adverse reaction was observed with a rate greater than 3%. | 1/2025 | |||||||||||||||||||||||
INVEGA TRINZA (paliperidone palmitate), a 3-month injection, is indicated for the treatment of schizophrenia in patients after they have been adequately treated with INVEGA SUSTENNA (1-month paliperidone palmitate extended-release injectable suspension) for at least four months
Adults
INVEGA TRINZA is to be used only after INVEGA SUSTENNA (1-month paliperidone palmitate extended-release injectable suspension) has been established as adequate treatment for at least four months. In order to establish a consistent maintenance dose, it is recommended that the last two doses of INVEGA SUSTENNA be the same dosage strength before starting INVEGA TRINZA.
Initiate INVEGA TRINZA when the next 1-month paliperidone palmitate dose is scheduled with an INVEGA TRINZA dose based on the previous 1-month injection dose, using the equivalent 3.5-fold higher dose as shown in Table 1. INVEGA TRINZA may be administered up to 7 days before or after the monthly time point of the next scheduled paliperidone palmitate 1-month dose.
| If the Last Dose of INVEGA SUSTENNA is: | Initiate INVEGA TRINZA at the Following Dose: |
|---|---|
| Conversion from the INVEGA SUSTENNA 39 mg dose was not studied. | |
| 78 mg | 273 mg |
| 117 mg | 410 mg |
| 156 mg | 546 mg |
| 234 mg | 819 mg |
Following the initial INVEGA TRINZA dose, INVEGA TRINZA should be administered every 3 months. If needed, dose adjustment can be made every 3 months in increments within the range of 273 mg to 819 mg based on individual patient tolerability and/or efficacy. Due to the long-acting nature of INVEGA TRINZA, the patient's response to an adjusted dose may not be apparent for several months
The efficacy of INVEGA TRINZA for the treatment of schizophrenia in patients who have been adequately treated for at least 4 months with INVEGA SUSTENNA (1-month paliperidone palmitate extended-release injectable suspension) was evaluated in a long-term double-blind, placebo-controlled randomized-withdrawal trial designed to evaluate time to relapse involving adult subjects who met DSM-IV-TR criteria for schizophrenia.
Patients could enter the study with acute symptoms (if previously treated with oral antipsychotics) or be clinically stable (if treated with long-acting injectable antipsychotics [LAI]). All patients who previously received oral antipsychotics received the paliperidone palmitate 1-month initiation regimen (deltoid injections of 234 mg and 156 mg one week apart), while those patients switching from LAI medication were treated with the 1-month paliperidone palmitate extended-release injectable suspension in place of the next scheduled injection. Specifically:
- For patients entering the study who were already being treated with the 1-month paliperidone palmitate extended-release injectable suspension, their dosing remained unchanged. Patients who were currently receiving the 39 mg dose of 1-month paliperidone palmitate were not eligible to enroll in the study.
- Patients entering the study who were being treated with 25 mg, 37.5 mg, or 50 mg of RISPERDAL CONSTA (risperidone long-acting injection) were switched to 78 mg, 117 mg, or 156 mg, respectively, of the 1-month paliperidone palmitate administered in the deltoid muscle.
- Patients entering the study who were being treated with any other LAI product were switched to 234 mg of the 1-month paliperidone palmitate administered in the deltoid muscle.
This study consisted of the following three treatment periods:
- A 17-week flexible-dose open-label period with the 1-month paliperidone palmitate (first part of a 29-week open-label stabilization phase). A total of 506 patients entered this phase of the study. Dosing of the 1-month paliperidone palmitate was individualized based on symptom response, tolerability, and previous medication history. Specifically, the dose could be adjusted at the week 5 and 9 injections and the injection site could be deltoid or gluteal. The week 13 dose had to be the same as the week 9 dose. Patients had to be clinically stable at the end of this period before receiving INVEGA TRINZA at the week 17 visit. Clinical stability was defined as achieving a PANSS total score <70 at week 17. The PANSS is a 30-item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); total PANSS scores range from 30 to 210.
- A 12-week open-label treatment period with INVEGA TRINZA (second part of a 29-week open-label stabilization phase). A total of 379 patients received a single-dose of INVEGA TRINZA which was a 3.5 multiple of the last dose of the 1-month paliperidone palmitate. Patients had to remain clinically stable before entry into the next period (double-blind). Clinical stability was defined as achieving a PANSS total score <70 and scores of ≤ 4 for seven specific PANSS items.
- A variable length double-blind treatment period. In this period, 305 stabilized patients were randomized 1:1 to continue treatment with INVEGA TRINZA or placebo until relapse, early withdrawal, or the end of study. Patients were randomized to the same dose of INVEGA TRINZA they received during the open-label phase (i.e., 273 mg, 410 mg, 546 mg, or 819 mg) or to placebo administered every 12 weeks. The numbers (%) of patients entering double-blind on each of the dose levels were 6 (4%) for 273 mg, 15 (9%) for 410 mg, 78 (49%) for 546 mg, and 61 (38%) for 819 mg.
The primary efficacy variable was time to first relapse. Relapse was pre-defined as emergence of one or more of the following: psychiatric hospitalization, ≥ 25% increase (if the baseline score was > 40) or a 10-point increase (if the baseline score was ≤ 40) in total PANSS score on two consecutive assessments, deliberate self-injury, violent behavior, suicidal/homicidal ideation, or a score of ≥ 5 (if the maximum baseline score was ≤ 3) or ≥ 6 (if the maximum baseline score was 4) on two consecutive assessments of the specific PANSS items.
A pre-planned interim analysis showed a statistically significantly longer time to relapse in patients treated with INVEGA TRINZA compared to placebo, and the study was stopped early because efficacy was demonstrated. The most common reason for relapse observed across both treatment groups was increase in the PANSS total score value, followed by psychiatric hospitalization.
Twenty-three percent (23%) of patients in the placebo group and 7.4% of patients in the INVEGA TRINZA group experienced a relapse event. The time to relapse was statistically significantly longer in patients randomized to the INVEGA TRINZA group than compared to placebo-treated patients. A Kaplan-Meier plot of time to relapse by treatment group is shown in Figure 4.
An examination of population subgroups did not reveal any clinically significant differences in responsiveness on the basis of gender, age, or race.
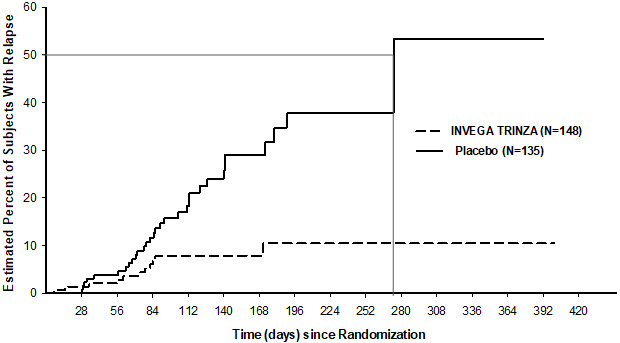
aThe median time to relapse in the placebo group was 274 days. The median time to relapse in the INVEGA TRINZA group could not be estimated due to low percentage (7.4%) of subjects with relapse.

- Use INVEGA TRINZA only after the patient has been adequately treated with the 1-month paliperidone palmitate extended-release injectable suspension for at least four months. ()
2.2 SchizophreniaAdults
INVEGA TRINZA is to be used only after INVEGA SUSTENNA (1-month paliperidone palmitate extended-release injectable suspension) has been established as adequate treatment for at least four months. In order to establish a consistent maintenance dose, it is recommended that the last two doses of INVEGA SUSTENNA be the same dosage strength before starting INVEGA TRINZA.
Initiate INVEGA TRINZA when the next 1-month paliperidone palmitate dose is scheduled with an INVEGA TRINZA dose based on the previous 1-month injection dose, using the equivalent 3.5-fold higher dose as shown in Table 1. INVEGA TRINZA may be administered up to 7 days before or after the monthly time point of the next scheduled paliperidone palmitate 1-month dose.
Table 1. INVEGA TRINZA Doses for Adult Patients Adequately Treated with INVEGA SUSTENNA If the Last Dose of INVEGA SUSTENNA is: Initiate INVEGA TRINZA at the Following Dose: Conversion from the INVEGA SUSTENNA 39 mg dose was not studied. 78 mg 273 mg 117 mg 410 mg 156 mg 546 mg 234 mg 819 mg Following the initial INVEGA TRINZA dose, INVEGA TRINZA should be administered every 3 months. If needed, dose adjustment can be made every 3 months in increments within the range of 273 mg to 819 mg based on individual patient tolerability and/or efficacy. Due to the long-acting nature of INVEGA TRINZA, the patient's response to an adjusted dose may not be apparent for several months
[see Clinical Pharmacology (12.3)]. - INVEGA TRINZA should be administered once every 3 months. ()
2.1 Administration InstructionsINVEGA TRINZA should be administered once every 3 months.
Each injection must be administered only by a healthcare professional.
Parenteral drug products should be inspected visually for foreign matter and discoloration prior to administration.
It is important to shake the syringe vigorously for at least 15 seconds to ensure a homogeneous suspension. Inject INVEGA TRINZA within 5 minutes of shaking vigorously[see Dosage and Administration (2.8)].INVEGA TRINZA is intended for intramuscular use only. Do not administer by any other route. Avoid inadvertent injection into a blood vessel. Administer the dose in a single injection; do not administer the dose in divided injections. Inject slowly, deep into the deltoid or gluteal muscle.
INVEGA TRINZA must be administered using only the thin wall needles that are provided in the INVEGA TRINZA pack. Do not use needles from the 1-month paliperidone palmitate extended-release injectable suspension pack or other commercially-available needles to reduce the risk of blockage.
Deltoid Injection
The recommended needle size for administration of INVEGA TRINZA into the deltoid muscle is determined by the patient's weight:
- For patients weighing less than 90 kg, the 1-inch, 22 gauge thin wall needle is recommended.
- For patients weighing 90 kg or more, the 1½-inch, 22 gauge thin wall needle is recommended.
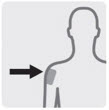
Administer into the center of the deltoid muscle. Deltoid injections should be alternated between the two deltoid muscles.
Gluteal Injection
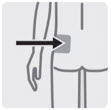
Regardless of patient weight, the recommended needle size for administration of INVEGA TRINZA into the gluteal muscle is the 1½-inch, 22 gauge thin wall needle. Administer into the upper-outer quadrant of the gluteal muscle. Gluteal injections should be alternated between the two gluteal muscles.
Incomplete Administration
To avoid an incomplete administration of INVEGA TRINZA, ensure that the prefilled syringe is
shaken vigorously for at least 15 seconds within 5 minutes prior to administration to ensure a homogeneous suspension and ensure the needle does not get clogged during injection[see Dosage and Administration (2.8)].However, in the event of an incompletely administered dose, do
notre-inject the dose remaining in the syringe and donotadminister another dose of INVEGA TRINZA. Closely monitor and treat the patient with oral supplementation as clinically appropriate until the next scheduled 3-month injection of INVEGA TRINZA. - For intramuscular injection only. ()
2.1 Administration InstructionsINVEGA TRINZA should be administered once every 3 months.
Each injection must be administered only by a healthcare professional.
Parenteral drug products should be inspected visually for foreign matter and discoloration prior to administration.
It is important to shake the syringe vigorously for at least 15 seconds to ensure a homogeneous suspension. Inject INVEGA TRINZA within 5 minutes of shaking vigorously[see Dosage and Administration (2.8)].INVEGA TRINZA is intended for intramuscular use only. Do not administer by any other route. Avoid inadvertent injection into a blood vessel. Administer the dose in a single injection; do not administer the dose in divided injections. Inject slowly, deep into the deltoid or gluteal muscle.
INVEGA TRINZA must be administered using only the thin wall needles that are provided in the INVEGA TRINZA pack. Do not use needles from the 1-month paliperidone palmitate extended-release injectable suspension pack or other commercially-available needles to reduce the risk of blockage.
Deltoid Injection
The recommended needle size for administration of INVEGA TRINZA into the deltoid muscle is determined by the patient's weight:
- For patients weighing less than 90 kg, the 1-inch, 22 gauge thin wall needle is recommended.
- For patients weighing 90 kg or more, the 1½-inch, 22 gauge thin wall needle is recommended.
Administer into the center of the deltoid muscle. Deltoid injections should be alternated between the two deltoid muscles.
Gluteal Injection
Regardless of patient weight, the recommended needle size for administration of INVEGA TRINZA into the gluteal muscle is the 1½-inch, 22 gauge thin wall needle. Administer into the upper-outer quadrant of the gluteal muscle. Gluteal injections should be alternated between the two gluteal muscles.
Incomplete Administration
To avoid an incomplete administration of INVEGA TRINZA, ensure that the prefilled syringe is
shaken vigorously for at least 15 seconds within 5 minutes prior to administration to ensure a homogeneous suspension and ensure the needle does not get clogged during injection[see Dosage and Administration (2.8)].However, in the event of an incompletely administered dose, do
notre-inject the dose remaining in the syringe and donotadminister another dose of INVEGA TRINZA. Closely monitor and treat the patient with oral supplementation as clinically appropriate until the next scheduled 3-month injection of INVEGA TRINZA. - Each injection must be administered only by a healthcare professional. ()
2.1 Administration InstructionsINVEGA TRINZA should be administered once every 3 months.
Each injection must be administered only by a healthcare professional.
Parenteral drug products should be inspected visually for foreign matter and discoloration prior to administration.
It is important to shake the syringe vigorously for at least 15 seconds to ensure a homogeneous suspension. Inject INVEGA TRINZA within 5 minutes of shaking vigorously[see Dosage and Administration (2.8)].INVEGA TRINZA is intended for intramuscular use only. Do not administer by any other route. Avoid inadvertent injection into a blood vessel. Administer the dose in a single injection; do not administer the dose in divided injections. Inject slowly, deep into the deltoid or gluteal muscle.
INVEGA TRINZA must be administered using only the thin wall needles that are provided in the INVEGA TRINZA pack. Do not use needles from the 1-month paliperidone palmitate extended-release injectable suspension pack or other commercially-available needles to reduce the risk of blockage.
Deltoid Injection
The recommended needle size for administration of INVEGA TRINZA into the deltoid muscle is determined by the patient's weight:
- For patients weighing less than 90 kg, the 1-inch, 22 gauge thin wall needle is recommended.
- For patients weighing 90 kg or more, the 1½-inch, 22 gauge thin wall needle is recommended.
Administer into the center of the deltoid muscle. Deltoid injections should be alternated between the two deltoid muscles.
Gluteal Injection
Regardless of patient weight, the recommended needle size for administration of INVEGA TRINZA into the gluteal muscle is the 1½-inch, 22 gauge thin wall needle. Administer into the upper-outer quadrant of the gluteal muscle. Gluteal injections should be alternated between the two gluteal muscles.
Incomplete Administration
To avoid an incomplete administration of INVEGA TRINZA, ensure that the prefilled syringe is
shaken vigorously for at least 15 seconds within 5 minutes prior to administration to ensure a homogeneous suspension and ensure the needle does not get clogged during injection[see Dosage and Administration (2.8)].However, in the event of an incompletely administered dose, do
notre-inject the dose remaining in the syringe and donotadminister another dose of INVEGA TRINZA. Closely monitor and treat the patient with oral supplementation as clinically appropriate until the next scheduled 3-month injection of INVEGA TRINZA. - For deltoid injection: For patients weighing less than 90 kg, use the 1-inch 22 gauge thin wall needle. For patients weighing 90 kg or more, use the 1½-inch 22 gauge thin wall needle.
- For gluteal injection: Regardless of patient weight, use the1½-inch 22 gauge thin wall needle.
- Prior to administration, shake the prefilled syringe vigorously for at least 15 seconds within 5 minutes prior to administration to ensure a homogeneous suspension. ()
2.1 Administration InstructionsINVEGA TRINZA should be administered once every 3 months.
Each injection must be administered only by a healthcare professional.
Parenteral drug products should be inspected visually for foreign matter and discoloration prior to administration.
It is important to shake the syringe vigorously for at least 15 seconds to ensure a homogeneous suspension. Inject INVEGA TRINZA within 5 minutes of shaking vigorously[see Dosage and Administration (2.8)].INVEGA TRINZA is intended for intramuscular use only. Do not administer by any other route. Avoid inadvertent injection into a blood vessel. Administer the dose in a single injection; do not administer the dose in divided injections. Inject slowly, deep into the deltoid or gluteal muscle.
INVEGA TRINZA must be administered using only the thin wall needles that are provided in the INVEGA TRINZA pack. Do not use needles from the 1-month paliperidone palmitate extended-release injectable suspension pack or other commercially-available needles to reduce the risk of blockage.
Deltoid Injection
The recommended needle size for administration of INVEGA TRINZA into the deltoid muscle is determined by the patient's weight:
- For patients weighing less than 90 kg, the 1-inch, 22 gauge thin wall needle is recommended.
- For patients weighing 90 kg or more, the 1½-inch, 22 gauge thin wall needle is recommended.
Administer into the center of the deltoid muscle. Deltoid injections should be alternated between the two deltoid muscles.
Gluteal Injection
Regardless of patient weight, the recommended needle size for administration of INVEGA TRINZA into the gluteal muscle is the 1½-inch, 22 gauge thin wall needle. Administer into the upper-outer quadrant of the gluteal muscle. Gluteal injections should be alternated between the two gluteal muscles.
Incomplete Administration
To avoid an incomplete administration of INVEGA TRINZA, ensure that the prefilled syringe is
shaken vigorously for at least 15 seconds within 5 minutes prior to administration to ensure a homogeneous suspension and ensure the needle does not get clogged during injection[see Dosage and Administration (2.8)].However, in the event of an incompletely administered dose, do
notre-inject the dose remaining in the syringe and donotadminister another dose of INVEGA TRINZA. Closely monitor and treat the patient with oral supplementation as clinically appropriate until the next scheduled 3-month injection of INVEGA TRINZA. - Initiate INVEGA TRINZA when the next 1-month paliperidone palmitate dose is scheduled with an INVEGA TRINZA dose based on the previous 1-month injection dose as shown below. ()
2.2 SchizophreniaAdults
INVEGA TRINZA is to be used only after INVEGA SUSTENNA (1-month paliperidone palmitate extended-release injectable suspension) has been established as adequate treatment for at least four months. In order to establish a consistent maintenance dose, it is recommended that the last two doses of INVEGA SUSTENNA be the same dosage strength before starting INVEGA TRINZA.
Initiate INVEGA TRINZA when the next 1-month paliperidone palmitate dose is scheduled with an INVEGA TRINZA dose based on the previous 1-month injection dose, using the equivalent 3.5-fold higher dose as shown in Table 1. INVEGA TRINZA may be administered up to 7 days before or after the monthly time point of the next scheduled paliperidone palmitate 1-month dose.
Table 1. INVEGA TRINZA Doses for Adult Patients Adequately Treated with INVEGA SUSTENNA If the Last Dose of INVEGA SUSTENNA is: Initiate INVEGA TRINZA at the Following Dose: Conversion from the INVEGA SUSTENNA 39 mg dose was not studied. 78 mg 273 mg 117 mg 410 mg 156 mg 546 mg 234 mg 819 mg Following the initial INVEGA TRINZA dose, INVEGA TRINZA should be administered every 3 months. If needed, dose adjustment can be made every 3 months in increments within the range of 273 mg to 819 mg based on individual patient tolerability and/or efficacy. Due to the long-acting nature of INVEGA TRINZA, the patient's response to an adjusted dose may not be apparent for several months
[see Clinical Pharmacology (12.3)].INVEGA TRINZA Doses for Adult Patients Adequately Treated with INVEGA SUSTENNA If the Last Dose of INVEGA SUSTENNA is: Initiate INVEGA TRINZA at the Following Dose: 78 mg 273 mg 117 mg 410 mg 156 mg 546 mg 234 mg 819 mg
Conversion from the INVEGA SUSTENNA 39 mg dose was not studied. - Missed Doses: Missing doses of INVEGA TRINZA should be avoided. To manage missed doses on exceptional occasions, refer to the Full Prescribing Information. ()
2.3 Missed DosesDosing Window
Missing doses of INVEGA TRINZA should be avoided. If necessary, patients may be given the injection up to 2 weeks before or after the 3-month time point.
Missed Dose 3½ Months to 4 Months Since Last Injection
If more than 3½ months (up to but less than 4 months) have elapsed since the last injection of INVEGA TRINZA, the previously administered INVEGA TRINZA dose should be administered as soon as possible, then continue with the 3-month injections following this dose.
Missed Dose 4 Months to 9 Months Since Last Injection
If 4 months up to and including 9 months have elapsed since the last injection of INVEGA TRINZA, do NOT administer the next dose of INVEGA TRINZA. Instead, use the re-initiation regimen shown in Table 2.
Table 2. Re-initiation Regimen After Missing 4 Months to 9 Months of INVEGA TRINZA If the Last Dose of INVEGA TRINZA was: Administer INVEGA SUSTENNA, two doses one week apart (into deltoid muscle) Then administer INVEGA TRINZA (into deltoidSee
Instructions for Use for deltoid injection needle selection based on body weight.or gluteal muscle)Day 1 Day 8 1 month after Day 8 273 mg 78 mg 78 mg 273 mg 410 mg 117 mg 117 mg 410 mg 546 mg 156 mg 156 mg 546 mg 819 mg 156 mg 156 mg 819 mg Missed Dose Longer than 9 Months Since Last Injection
If more than 9 months have elapsed since the last injection of INVEGA TRINZA, re-initiate treatment with the 1-month paliperidone palmitate extended-release injectable suspension as described in the prescribing information for that product. INVEGA TRINZA can then be resumed after the patient has been adequately treated with the 1-month paliperidone palmitate extended-release injectable suspension for at least 4 months.
- Moderate to severe renal impairment (creatinine clearance < 50 mL/min): INVEGA TRINZA is not recommended. ()
2.5 Dosage Recommendations in Patients with Renal ImpairmentINVEGA TRINZA has not been systematically studied in patients with renal impairment[see Clinical Pharmacology (12.3)]. For patients with mild renal impairment (creatinine clearance ≥ 50 mL/min to < 80 mL/min [Cockcroft-Gault Formula]), adjust dosage and stabilize the patient using the 1-month paliperidone palmitate extended-release injectable suspension, then transition to INVEGA TRINZA(see Table 1) [see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Refer to the Prescribing Information of the 1-month paliperidone palmitate extended-release injectable suspension product for the recommended dosage in patients with mild renal impairment.INVEGA TRINZA is not recommended in patients with moderate or severe renal impairment (creatinine clearance < 50 mL/min)
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)]. - Mild renal impairment (creatinine clearance ≥ 50 mL/min to < 80 mL/min): Adjust dosage and stabilize the patient using INVEGA SUSTENNA, then transition to INVEGA TRINZA. See above. (
INVEGA TRINZA Doses for Adult Patients Adequately Treated with INVEGA SUSTENNA If the Last Dose of INVEGA SUSTENNA is: Initiate INVEGA TRINZA at the Following Dose: 78 mg 273 mg 117 mg 410 mg 156 mg 546 mg 234 mg 819 mg )2.5 Dosage Recommendations in Patients with Renal ImpairmentINVEGA TRINZA has not been systematically studied in patients with renal impairment[see Clinical Pharmacology (12.3)]. For patients with mild renal impairment (creatinine clearance ≥ 50 mL/min to < 80 mL/min [Cockcroft-Gault Formula]), adjust dosage and stabilize the patient using the 1-month paliperidone palmitate extended-release injectable suspension, then transition to INVEGA TRINZA(see Table 1) [see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Refer to the Prescribing Information of the 1-month paliperidone palmitate extended-release injectable suspension product for the recommended dosage in patients with mild renal impairment.INVEGA TRINZA is not recommended in patients with moderate or severe renal impairment (creatinine clearance < 50 mL/min)
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].
INVEGA TRINZA is available as a white to off-white aqueous extended-release injectable suspension for intramuscular injection in dose strengths of 273 mg/0.88 mL, 410 mg/1.32 mL, 546 mg/1.75 mL, and 819 mg/2.63 mL paliperidone palmitate in single-dose prefilled syringes.
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including INVEGA TRINZA, during pregnancy. Healthcare providers are encouraged to register patients by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or online at
Risk Summary
Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
In animal reproduction studies, there were no treatment related effects on the offspring when pregnant rats were injected intramuscularly with paliperidone palmitate during the period of organogenesis at doses up to 10 times the maximum recommended human dose (MRHD) of 234 mg paliperidone based on mg/m2body surface area. There were no increases in fetal abnormalities when pregnant rats and rabbits were treated orally with paliperidone during the period of organogenesis with up to 8 times the MRHD of 12 mg of paliperidone based on mg/m2body surface area. Additional reproduction toxicity studies were conducted with orally administered risperidone, which is extensively converted to paliperidone (see Animal data).
Clinical Considerations
There is a risk to the mother from untreated schizophrenia, including increased risk of relapse, hospitalization, and suicide. Schizophrenia is associated with increased adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs, including INVEGA TRINZA, during the third trimester of pregnancy. These symptoms have varied in severity. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization.
Data
Published data from observational studies, birth registries, and case reports on the use of atypical antipsychotics during pregnancy do not report a clear association with antipsychotics and major birth defects. A prospective observational study including 6 women treated with risperidone, the parent compound of paliperidone, demonstrated placental passage of risperidone and paliperidone. A retrospective cohort study from a Medicaid database of 9258 women exposed to antipsychotics during pregnancy did not indicate an overall increased risk for major birth defects. There was a small increase in the risk of major birth defects (RR=1.26, 95% CI 1.02–1.56) and of cardiac malformations (RR=1.26, 95% CI 0.88–1.81) in a subgroup of 1566 women exposed to the parent compound of paliperidone, risperidone, during the first trimester of pregnancy; however, there is no mechanism of action to explain the difference in malformation rates.
No developmental toxicity studies were conducted with the 3-month paliperidone palmitate extended-release injectable suspension.
There were no treatment-related effects on the offspring when pregnant rats were injected intramuscularly with 1-month paliperidone palmitate extended-release injectable suspension during the period of organogenesis at doses up to 250 mg/kg, which is 3 times the MRHD of 819 mg of the 3-month paliperidone palmitate extended-release injectable suspension based on mg/m2body surface area.
In animal reproduction studies, there were no increases in fetal abnormalities when pregnant rats and rabbits were treated orally with paliperidone during the period of organogenesis with up to 8 times the MRHD of 12 mg based on mg/m2body surface area.
Additional reproduction toxicity studies were conducted with orally administered risperidone, which is extensively converted to paliperidone. Cleft palate was observed in the offspring of pregnant mice treated with risperidone at 3 to 4 times the MRHD of 16 mg based on mg/m2body surface area; maternal toxicity occurred at 4 times the MHRD. There was no evidence of teratogenicity in embryo-fetal developmental toxicity studies with risperidone in rats and rabbits at doses up to 6 times the MRHD of 16 mg/day risperidone based on mg/m2body surface area. When the offspring of pregnant rats, treated with risperidone at 0.6 times the MRHD based on mg/m2body surface area, reached adulthood, learning was impaired. Increased neuronal cell death occurred in the fetal brains of the offspring of pregnant rats treated at 0.5 to 1.2 times the MRHD; the postnatal development and growth of the offspring was delayed.
In rat reproduction studies with risperidone, pup deaths occurred at oral doses which are less than the MRHD of risperidone based on mg/m2body surface area; it is not known whether these deaths were due to a direct effect on the fetuses or pups or, to effects on the dams (see RISPERDAL package insert).