Iopamidol
Iopamidol Prescribing Information
Iopamidol Injection is indicated for intrathecal administration in adult neuroradiology including myelography (lumbar, thoracic, cervical, total columnar), and for contrast enhancement of computed tomographic (CECT) cisternography and ventriculography. Iopamidol Injection, 41% is indicated for thoraco-lumbar myelography in children over the age of two years.
In
The usual recommended adult dose range for iopamidol is 2000-3000 mg iodine. Iopamidol formulated to contain more than 300 mgI/mL should not be used intrathecally in adults. The minimum dose needed to perform a procedure should always be used.
In
The usual recommended pediatric dose range for iopamidol is 1400-2400 mg iodine. Iopamidol formulated to contain more than 200 mgI/mL should not be used intrathecally in children. The minimum dose needed to perform a procedure should always be used. See
Anesthesia is not necessary. However, young children may require general anesthesia for technical reasons. Premedication with sedatives or tranquillizers is usually not needed. In patients with a history of seizure activity who are not on anticonvulsant therapy, premedication with barbiturates or phenytoin should be considered.
Lumbar puncture is usually made between L3 and L4; if pathology is suspected at this level, the interspace immediately above or below may be selected. A lateral cervical puncture may also be used.
An interval of at least 48 hours should be allowed before repeat examination; however, whenever possible five to seven days is recommended.
The pediatric doses listed below, intended as a guideline, are based on age rather than weight because the brain and CSF capacity is independent of weight. Variations will depend on such factors as height, suspected pathology, the patient’s condition, technique used, etc. (e.g. CT or standard radiology or movement of the contrast media directed distal to the site of injection).
Pediatric Dosage Table | ||
Iopamidol Injection, 41% (200 mgI/mL) | ||
Procedure | Age Years | Usual Recommended Dose (mL) |
Lumbar, thoracic myelogram | 2-7 | 7-9 |
8-12 | 8-11 | |
13-18 | 10-12 | |
Adult Dosage Table | ||
Concentration of Solution (mgI/mL) | Usual Recommended Dose (mL) | |
Lumbar myelogram | 200 | 10 to 15 |
Thoracic myelogram | 200 | 10 to 15 |
Cervical myelogram | 200 | 10 to 15 |
(via lumbar injection) | 300 | 10 |
Cervical myelogram (via lateral cervical injection) | 200 | 10 |
Total columnar myelography | 300 | 10 |
CT cisternography (via lumbar injection) | 200 | 4 to 6 |
Following subarachnoid injection, conventional radiography will continue to provide good diagnostic contrast for at least 30 minutes. At about one hour, diagnostic degree of contrast will not usually be available. However, sufficient contrast for CT myelography will be available for several hours. CT myelography following conventional myelography should be deferred for at least four hours to reduce the degree of contrast. Aspiration of iopamidol is unnecessary following intrathecal administration (see
The pharmacokinetics of intravenously administered iopamidol in normal subjects conform to an open two-compartment model with first order elimination (a rapid alpha phase for drug distribution and a slow beta phase for drug elimination). The elimination serum or plasma half-life is approximately two hours; the half-life is not dose dependent. No significant metabolism, deiodination, or biotransformation occurs.
Iopamidol is rapidly absorbed into the bloodstream from cerebrospinal fluid (CSF); following intrathecal administration, iopamidol appears in plasma within one hour and virtually all of the drug reaches the systemic circulation within 24 hours. Iopamidol is excreted mainly through the kidneys following intrathecal administration, and the drug is essentially undetectable in the plasma 48 hours later. In patients with impaired renal function, the elimination half-life is prolonged dependent upon the degree of impairment. In the absence of renal dysfunction, the cumulative urinary excretion for iopamidol, expressed as a percentage of administered intravenous dose is approximately 35 to 40 percent at 60 minutes, 80 to 90 percent at 8 hours, and 90 percent or more in the 72- to 96-hour period after administration. In normal subjects, approximately 1 percent or less of the administered dose appears in cumulative 72- to 96-hour fecal specimens.
Iopamidol displays little tendency to bind to serum or plasma proteins.
No evidence of
Animal studies indicate that iopamidol does not cross the blood-brain barrier to any significant extent following intravascular administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Iopamidol solutions should be used only if clear and within the normal colorless to pale yellow range. Discard any product which shows signs of crystallization or damage to the container-closure system, which includes the glass container, stopper and/or crimp.
It is desirable that solutions of radiopaque diagnostic agents for intrathecal use be at body temperature when injected. Withdrawal of contrast agents from their containers should be accomplished under aseptic conditions with sterile syringes. Spinal puncture must always be performed under sterile conditions.
Intrathecal administration of corticosteroids with iopamidol is contraindicated. Because of overdosage considerations, immediate repeat myelography in the event of technical failure is contraindicated (see interval recommendation under
In
The usual recommended adult dose range for iopamidol is 2000-3000 mg iodine. Iopamidol formulated to contain more than 300 mgI/mL should not be used intrathecally in adults. The minimum dose needed to perform a procedure should always be used.
In
The usual recommended pediatric dose range for iopamidol is 1400-2400 mg iodine. Iopamidol formulated to contain more than 200 mgI/mL should not be used intrathecally in children. The minimum dose needed to perform a procedure should always be used. See
Anesthesia is not necessary. However, young children may require general anesthesia for technical reasons. Premedication with sedatives or tranquillizers is usually not needed. In patients with a history of seizure activity who are not on anticonvulsant therapy, premedication with barbiturates or phenytoin should be considered.
Lumbar puncture is usually made between L3 and L4; if pathology is suspected at this level, the interspace immediately above or below may be selected. A lateral cervical puncture may also be used.
An interval of at least 48 hours should be allowed before repeat examination; however, whenever possible five to seven days is recommended.
The pediatric doses listed below, intended as a guideline, are based on age rather than weight because the brain and CSF capacity is independent of weight. Variations will depend on such factors as height, suspected pathology, the patient’s condition, technique used, etc. (e.g. CT or standard radiology or movement of the contrast media directed distal to the site of injection).
Pediatric Dosage Table | ||
Iopamidol Injection, 41% (200 mgI/mL) | ||
Procedure | Age Years | Usual Recommended Dose (mL) |
Lumbar, thoracic myelogram | 2-7 | 7-9 |
8-12 | 8-11 | |
13-18 | 10-12 | |
Adult Dosage Table | ||
Concentration of Solution (mgI/mL) | Usual Recommended Dose (mL) | |
Lumbar myelogram | 200 | 10 to 15 |
Thoracic myelogram | 200 | 10 to 15 |
Cervical myelogram | 200 | 10 to 15 |
(via lumbar injection) | 300 | 10 |
Cervical myelogram (via lateral cervical injection) | 200 | 10 |
Total columnar myelography | 300 | 10 |
CT cisternography (via lumbar injection) | 200 | 4 to 6 |
Following subarachnoid injection, conventional radiography will continue to provide good diagnostic contrast for at least 30 minutes. At about one hour, diagnostic degree of contrast will not usually be available. However, sufficient contrast for CT myelography will be available for several hours. CT myelography following conventional myelography should be deferred for at least four hours to reduce the degree of contrast. Aspiration of iopamidol is unnecessary following intrathecal administration (see CLINICAL PHARMACOLOGY).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Iopamidol solutions should be used only if clear and within the normal colorless to pale yellow range. Discard any product which shows signs of crystallization or damage to the container-closure system, which includes the glass container, stopper and/or crimp.
It is desirable that solutions of radiopaque diagnostic agents for intrathecal use be at body temperature when injected. Withdrawal of contrast agents from their containers should be accomplished under aseptic conditions with sterile syringes. Spinal puncture must always be performed under sterile conditions.
- See WARNINGSregarding discontinuation of neuroleptic agents.
- Maintain normal diet up to 2 hours before procedure.
- Ensure hydration-fluids up to time of procedure.
- Use minimum dose and concentration required for satisfactory contrast.
- Inject slowly over 1 to 2 minutes to avoid excessive mixing.
- Abrupt or active patient movement causes excessive mixing with CSF.
- Instruct patient to remainpassive. Move patientslowlyand only as necessary.
- To maintain as a bolus, move medium to distal areavery slowlyunder fluoroscopic control.
- In all positioning techniques keep the patient’s head elevated above highest level of spine.
- Do not lower head of table more than 15° during thoraco-cervical procedures.
- In patients with excessive lordosis, consider lateral position for injection and movement of the medium cephalad.
- Avoid intracranial entry of a bolus.
- Avoid early and high cephalad dispersion of the medium.
- At completion of direct cervical or lumbo-cervical procedures, raise head of table steeply (45°) for about 2 minutes to restore medium to lower levels.
- Raise head of stretcher to at least 30° before moving patient onto it.
- Movement onto stretcher, and off the stretcher to bed, should be done slowly with patient completely passive, maintaininghead upposition.
- Before moving patient onto bed, raise head of bed 30° to 45° and maintain the patient in this position under close observation for 12 to 24 hours.
- Advise patient to remain still in bed, inhead upposition for the first 24 hours.
- Obtain visitors cooperation in keeping the patient quiet and inhead upposition, especially in first few hours.
- Encourage oral fluids and diet as tolerated.
- Antinauseants of the phenothiazine class should not be administered to treat postprocedural nausea or vomiting (see WARNINGS). Since persistent nausea and vomiting may result in dehydration, prompt consideration of volume replacement by intravenous fluids is recommended.
Many radiopaque contrast agents are incompatible
The most frequently reported adverse reactions following intrathecal administration of iopamidol are headache, nausea, vomiting, and musculoskeletal pain. These reactions usually occur 1 to 10 hours after injection, almost all occurring within 24 hours. They are usually mild to moderate in degree, lasting for a few hours and usually disappearing within 24 hours. Rarely, headaches may be severe or persist for days. Headache is often accompanied by nausea and vomiting, and tends to be more frequent and persistent in patients not optimally hydrated. Backache, neck stiffness, numbness and paresthesias, leg or sciatic-type pain occurred less frequently, often in the form of a transient exacerbation of pre-existing symptomatology. Transient alterations in vital signs may occur and their significance must be assessed on an individual basis.
The following table of incidence of reactions is based on clinical studies with Iopamidol Injection in about 686 patients.
Adverse Reactions | ||
Estimated Overall Incidence | ||
System |
| ≤ 1% |
Body as a Whole | headache (16.4%) | pyrexia |
muscle weakness | ||
hot flashes | ||
malaise | ||
fatigue | ||
weakness | ||
Digestive | nausea (7.3%) | diarrhea |
vomiting (3.6%) | heartburn | |
Musculoskeletal | back pain (2.2%) | leg cramps |
leg pain (1.4%) | sciatica | |
neck pain (1.1%) | cervicobrachial irritation | |
meningeal irritation | ||
radicular irritation lumbosacral | ||
other musculoskeletal pain | ||
involuntary movement | ||
burning sensation | ||
Cardiovascular | hypotension (1.1%) | tachycardia |
hypertension | ||
chest pain | ||
Nervous | none | emotional stress |
dizziness | ||
paresthesia | ||
confusion | ||
hallucinations | ||
lightheadedness | ||
syncope | ||
numbness | ||
cold extremities | ||
ataxia | ||
irritability | ||
Urogenital | none | urinary retention |
Respiratory | none | dyspnea |
Skin and Appendages | none | rash |
Miscellaneous | none | injection site pain |
Other adverse effects reported in clinical literature for iopamidol include facial neuralgia, tinnitus, and sweating.
Major motor seizures have been reported in the clinical literature and since market introduction in the United States. Early onset of seizures (less than two hours) is indicative of early substantial intracranial entry. Transitory EEG changes occur and usually take the form of slow wave activity.
While not observed in controlled clinical studies with Iopamidol Injection, the following adverse reactions may occur because they have been reported with Iopamidol Injection and other nonionic water soluble contrast agents: cardiovascular (arrhythmias); pulmonary (apnea); bacterial meningitis, and aseptic meningitis syndrome; allergy or idiosyncrasy (chills, pruritus, nasal congestion, Guillain-Barre syndrome); CNS irritation (psycho-organic syndrome: mild and transitory perceptual aberrations such as depersonalization, anxiety, depression, hyperesthesia, disturbances in speech, sight, or hearing, and disorientation; in addition, hyperreflexia or areflexia, hypertonia or flaccidity, restlessness, tremor, echoacousia, echolalia, asterixis or dysphasia have occurred). Profound mental disturbances have rarely been reported (various forms and degrees of aphasia, mental confusion or disorientation); the onset is usually at 8 to 10 hours and lasts for about 24 hours without aftereffects. However, occasionally they have been manifest as apprehension, agitation or progressive withdrawal to the point of stupor or coma. In a few cases, these have been accompanied by transitory hearing loss or other auditory symptoms and visual disturbances (believed subjective or delusional). Persistent cortical loss of vision in association with convulsions, and ventricular block have been reported. Rarely, persistent though transitory weakness in the leg or ocular muscles has been reported.
Other drugs should not be admixed with iopamidol (see
Intrathecal administration of corticosteroids with iopamidol is contraindicated. Because of overdosage considerations, immediate repeat myelography in the event of technical failure is contraindicated (see interval recommendation under DOSAGE AND ADMINISTRATION). Myelography should not be performed in the presence of significant local or systemic infection where bacteremia is likely.
In
The usual recommended adult dose range for iopamidol is 2000-3000 mg iodine. Iopamidol formulated to contain more than 300 mgI/mL should not be used intrathecally in adults. The minimum dose needed to perform a procedure should always be used.
In
The usual recommended pediatric dose range for iopamidol is 1400-2400 mg iodine. Iopamidol formulated to contain more than 200 mgI/mL should not be used intrathecally in children. The minimum dose needed to perform a procedure should always be used. See
Anesthesia is not necessary. However, young children may require general anesthesia for technical reasons. Premedication with sedatives or tranquillizers is usually not needed. In patients with a history of seizure activity who are not on anticonvulsant therapy, premedication with barbiturates or phenytoin should be considered.
Lumbar puncture is usually made between L3 and L4; if pathology is suspected at this level, the interspace immediately above or below may be selected. A lateral cervical puncture may also be used.
An interval of at least 48 hours should be allowed before repeat examination; however, whenever possible five to seven days is recommended.
The pediatric doses listed below, intended as a guideline, are based on age rather than weight because the brain and CSF capacity is independent of weight. Variations will depend on such factors as height, suspected pathology, the patient’s condition, technique used, etc. (e.g. CT or standard radiology or movement of the contrast media directed distal to the site of injection).
Pediatric Dosage Table | ||
Iopamidol Injection, 41% (200 mgI/mL) | ||
Procedure | Age Years | Usual Recommended Dose (mL) |
Lumbar, thoracic myelogram | 2-7 | 7-9 |
8-12 | 8-11 | |
13-18 | 10-12 | |
Adult Dosage Table | ||
Concentration of Solution (mgI/mL) | Usual Recommended Dose (mL) | |
Lumbar myelogram | 200 | 10 to 15 |
Thoracic myelogram | 200 | 10 to 15 |
Cervical myelogram | 200 | 10 to 15 |
(via lumbar injection) | 300 | 10 |
Cervical myelogram (via lateral cervical injection) | 200 | 10 |
Total columnar myelography | 300 | 10 |
CT cisternography (via lumbar injection) | 200 | 4 to 6 |
Following subarachnoid injection, conventional radiography will continue to provide good diagnostic contrast for at least 30 minutes. At about one hour, diagnostic degree of contrast will not usually be available. However, sufficient contrast for CT myelography will be available for several hours. CT myelography following conventional myelography should be deferred for at least four hours to reduce the degree of contrast. Aspiration of iopamidol is unnecessary following intrathecal administration (see CLINICAL PHARMACOLOGY).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Iopamidol solutions should be used only if clear and within the normal colorless to pale yellow range. Discard any product which shows signs of crystallization or damage to the container-closure system, which includes the glass container, stopper and/or crimp.
It is desirable that solutions of radiopaque diagnostic agents for intrathecal use be at body temperature when injected. Withdrawal of contrast agents from their containers should be accomplished under aseptic conditions with sterile syringes. Spinal puncture must always be performed under sterile conditions.
- See WARNINGSregarding discontinuation of neuroleptic agents.
- Maintain normal diet up to 2 hours before procedure.
- Ensure hydration-fluids up to time of procedure.
- Use minimum dose and concentration required for satisfactory contrast.
- Inject slowly over 1 to 2 minutes to avoid excessive mixing.
- Abrupt or active patient movement causes excessive mixing with CSF.
- Instruct patient to remainpassive. Move patientslowlyand only as necessary.
- To maintain as a bolus, move medium to distal areavery slowlyunder fluoroscopic control.
- In all positioning techniques keep the patient’s head elevated above highest level of spine.
- Do not lower head of table more than 15° during thoraco-cervical procedures.
- In patients with excessive lordosis, consider lateral position for injection and movement of the medium cephalad.
- Avoid intracranial entry of a bolus.
- Avoid early and high cephalad dispersion of the medium.
- At completion of direct cervical or lumbo-cervical procedures, raise head of table steeply (45°) for about 2 minutes to restore medium to lower levels.
- Raise head of stretcher to at least 30° before moving patient onto it.
- Movement onto stretcher, and off the stretcher to bed, should be done slowly with patient completely passive, maintaininghead upposition.
- Before moving patient onto bed, raise head of bed 30° to 45° and maintain the patient in this position under close observation for 12 to 24 hours.
- Advise patient to remain still in bed, inhead upposition for the first 24 hours.
- Obtain visitors cooperation in keeping the patient quiet and inhead upposition, especially in first few hours.
- Encourage oral fluids and diet as tolerated.
- Antinauseants of the phenothiazine class should not be administered to treat postprocedural nausea or vomiting (see WARNINGS). Since persistent nausea and vomiting may result in dehydration, prompt consideration of volume replacement by intravenous fluids is recommended.
Many radiopaque contrast agents are incompatible
Many radiopaque contrast agents are incompatible
Iopamidol Injection, USP formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intrathecal administration.
Each mL of Iopamidol Injection, USP, 41% provides 408 mg iopamidol with 1 mg tromethamine and 0.26 mg edetate calcium disodium. The solution contains approximately 0.029 mg (0.001 mEq) sodium and 200 mg organically bound iodine per mL.
Each mL of Iopamidol Injection, USP, 61% provides 612 mg iopamidol with 1 mg tromethamine and 0.39 mg edetate calcium disodium. The solution contains approximately 0.043 mg (0.002 mEq) sodium and 300 mg organically bound iodine per mL.
The pH of Iopamidol Injection, USP contrast media has been adjusted to 6.5-7.5 with hydrochloric acid and/or sodium hydroxide. Pertinent physicochemical data are noted below. Iopamidol Injection, USP is hypertonic as compared to plasma and cerebrospinal fluid (approximately 285 and 301 mOsm/kg water, respectively).
Iopamidol | ||
Parameter | 41% | 61% |
Concentration (mgI/mL) | 200 | 300 |
Osmolality @ 37°C (mOsm/kg water) | 413 | 616 |
Viscosity (cP) @ 37°C | 2.0 | 4.7 |
@ 20°C | 3.3 | 8.8 |
Specific Gravity @ 37°C | 1.216 | 1.328 |
Iopamidol is designated chemically as (S)-N,N’-bis[2-hydroxy-1-(hydroxymethyl)-ethyl]- 2,4,6-triiodo-5-lactamidoisophthalamide. Structural formula:
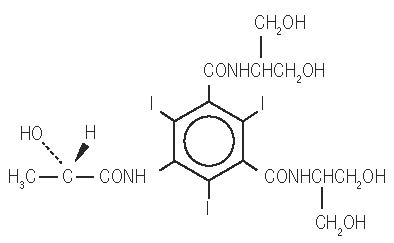
MW 777.09
C
17H
22I
3N
3O
8
CAS-60166-93-0
Organically Bound Iodine: 49%