Ipratropium Bromide
Ipratropium Bromide Prescribing Information
Ipratropium Bromide Inhalation Solution administered either alone or with other bronchodilators, especially beta adrenergics, is indicated as a bronchodilator for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema.
The usual dosage of ipratropium bromide inhalation solution is 500 mcg (1 Unit-Dose Vial) administered three to four times a day by oral nebulization, with doses 6 to 8 hours apart. Ipratropium bromide inhalation solution unit-dose vials contain 500 mcg ipratropium bromide, USP anhydrous in 2.5 mL normal saline. Ipratropium bromide inhalation solution can be mixed in the nebulizer with albuterol or metaproterenol if used within one hour. Drug stability and safety of Ipratropium Bromide Inhalation Solution when mixed with other drugs in a nebulizer have not been established.
Ipratropium bromide is contraindicated in known or suspected cases of hypersensitivity to ipratropium bromide, or to atropine and its derivatives.
Adverse reaction information concerning ipratropium bromide inhalation solution is derived from 12-week active-controlled clinical trials. Additional information is derived from foreign post-marketing experience and the published literature.
All adverse events, regardless of drug relationship, reported by three percent or more patients in the 12-week controlled clinical trials appear in the table below.
Additional adverse reactions reported in less than three percent of the patients treated with ipratropium bromide include tachycardia, palpitations, eye pain, urinary retention, urinary tract infection and urticaria. Cases of precipitation or worsening of narrow-angle glaucoma, mydriasis, and acute eye pain have been reported.
Lower respiratory adverse reactions (bronchitis, dyspnea and bronchospasm) were the most common events leading to discontinuation of ipratropium bromide therapy in the 12-week trials. Headache, mouth dryness and aggravation of COPD symptoms are more common when the total daily dose of ipratropium bromide equals or exceeds 2,000 mcg.
Allergic-type reactions such as skin-rash, angioedema of tongue, lips and face, urticaria, laryngospasm and anaphylactic reaction have been reported. Many of the patients had a history of allergies to other drugs and/or foods.
| PERCENT OF PATIENTS | |||||
|---|---|---|---|---|---|
Ipratropium | Metaproterenol | Ipratropium/ Metaproterenol | Albuterol | Ipratropium/ Albuterol | |
| (500 mcg t.i.d.) | (15 mg t.i.d.) | (500 mcg t.i.d./ 15 mg t.i.d.) | (2.5 mg t.i.d.) | (500 mcg t.i.d./ 2.5 mg t.i.d.) | |
| n = 219 | n = 212 | n = 108 | n = 205 | n = 100 | |
Body as a Whole-General Disorders | |||||
| Headache | 6.4 | 5.2 | 6.5 | 6.3 | 9.0 |
| Pain | 4.1 | 3.3 | 0.9 | 2.9 | 5.0 |
| Influenza-like symptoms | 3.7 | 4.7 | 6.5 | 0.5 | 1.0 |
| Back Pain | 3.2 | 1.9 | 1.9 | 2.4 | 0.0 |
| Chest Pain | 3.2 | 4.2 | 5.6 | 2.0 | 1.0 |
Cardiovascular Disorders | |||||
| Hypertension/Hypertension Aggravated | 0.9 | 1.9 | 0.9 | 1.5 | 4.0 |
Central & Peripheral Nervous System | |||||
| Dizziness | 2.3 | 3.3 | 1.9 | 3.9 | 4.0 |
| Insomnia | 0.9 | 0.5 | 4.6 | 1.0 | 1.0 |
| Tremor | 0.9 | 7.1 | 8.3 | 1.0 | 0.0 |
| Nervousness | 0.5 | 4.7 | 6.5 | 1.0 | 1.0 |
Gastrointestinal System Disorders | |||||
| Mouth Dryness | 3.2 | 0.0 | 1.9 | 2.0 | 3.0 |
| Nausea | 4.1 | 3.8 | 1.9 | 2.9 | 2.0 |
| Constipation | 0.9 | 0.0 | 3.7 | 1.0 | 1.0 |
Musculo-skeletal System Disorders | |||||
| Arthritis | 0.9 | 1.4 | 0.9 | 0.5 | 3.0 |
Respiratory System Disorders (Lower) | |||||
| Coughing | 4.6 | 8.0 | 6.5 | 5.4 | 6.0 |
| Dyspnea | 9.6 | 13.2 | 16.7 | 12.7 | 9.0 |
| Bronchitis | 14.6 | 24.5 | 15.7 | 16.6 | 20.0 |
| Bronchospasm | 2.3 | 2.8 | 4.6 | 5.4 | 5.0 |
| Sputum Increased | 1.4 | 1.4 | 4.6 | 3.4 | 0.0 |
| Respiratory Disorder | 0.0 | 6.1 | 6.5 | 2.0 | 4.0 |
Respiratory System Disorders (Upper) | |||||
| Upper Respiratory Tract Infection | 13.2 | 11.3 | 9.3 | 12.2 | 16.0 |
| Pharyngitis | 3.7 | 4.2 | 5.6 | 2.9 | 4.0 |
| Rhinitis | 2.3 | 4.2 | 1.9 | 2.4 | 0.0 |
| Sinusitus | 2.3 | 2.8 | 0.9 | 5.4 | 4.0 |
Ipratropium bromide has been shown to be a safe and effective bronchodilator when used in conjunction with beta adrenergic bronchodilators. Ipratropium bromide has also been used with other pulmonary medications, including methylxanthines and corticosteroids, without adverse drug interactions.
The active ingredient, ipratropium bromide monohydrate, USP, is an anticholinergic bronchodilator chemically described as 8-azoniabicyclo [3.2.1]- octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide, monohydrate (endo, syn)-, (±)-; a synthetic quaternary ammonium compound, chemically related to atropine.
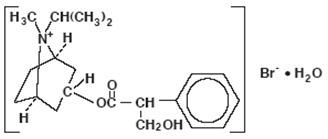
Ipratropium Bromide Monohydrate C
20H
30BrNO
3•H
2O Mol.Wt. 430.4
Ipratropium bromide is a white crystalline substance, freely soluble in water and lower alcohols. It is a quaternary ammonium compound and thus exists in an ionized state in aqueous solutions. It is relatively insoluble in non-polar media.
Ipratropium Bromide Inhalation Solution is administered by oral inhalation with the aid of a nebulizer. It contains ipratropium bromide, USP 0.02% (anhydrous basis) in a sterile, preservative-free, isotonic saline solution, pH-adjusted to 3.4 (3 to 4) with hydrochloric acid.