Ipratropium Bromide
Ipratropium Bromide Prescribing Information
Ipratropium bromide nasal solution, 0.03% is indicated for the symptomatic relief of rhinorrhea associated with allergic and nonallergic perennial rhinitis in adults and children age 6 years and older. Ipratropium bromide nasal solution, 0.03% does not relieve nasal congestion, sneezing, or postnasal drip associated with allergic or nonallergic perennial rhinitis.
The recommended dose of ipratropium bromide nasal solution, 0.03% is two sprays (42 mcg) per nostril two or three times daily (total dose 168 to 252 mcg/day) for the symptomatic relief of rhinorrhea associated with allergic and nonallergic perennial rhinitis in adults and children age 6 years and older. Optimum dosage varies with the response of the individual patient.
Initial pump priming requires seven sprays of the pump. If used regularly as recommended, no further priming is required. If not used for more than 24 hours, the pump will require two sprays, or if not used for more than seven days, the pump will require seven sprays to reprime.
Ipratropium bromide nasal solution, 0.03% is contraindicated in patients with a history of hypersensitivity to atropine or its derivatives, or to any of the other ingredients.
Adverse reaction information on ipratropium bromide nasal solution, 0.03% in patients with perennial rhinitis was derived from four multicenter, vehicle-controlled clinical trials involving 703 patients (356 patients on ipratropium bromide and 347 patients on vehicle), and a one-year, open-label, follow-up trial. In three of the trials, patients received ipratropium bromide nasal solution, 0.03% three times daily, for eight weeks. In the other trial, ipratropium bromide nasal solution, 0.03% was given to patients two times daily for four weeks. Of the 285 patients who entered the open-label, follow-up trial, 232 were treated for 3 months, 200 for 6 months, and 159 up to one year. The majority (>86%) of patients treated for one year were maintained on 42 mcg per nostril, two or three times daily, of ipratropium bromide nasal solution, 0.03%.
Table 1 shows adverse events, and the frequency that these adverse events led to the discontinuation of treatment, reported for patients who received ipratropium bromide nasal solution, 0.03% at the recommended dose of 42 mcg per nostril, or vehicle two or three times daily for four or eight weeks. Only adverse events reported with an incidence of at least 2.0% in the ipratropium bromide group and higher in the ipratropium bromide group than in the vehicle group are shown.
Ipratropium bromide | Vehicle Control | |||
Nasal Spray 0.03% | ||||
(n=356) | (n=347) | |||
Incidence % | Discontinued % | Incidence % | Discontinued % | |
+ This table includes adverse events which occurred at an incidence rate of at least 2.0% in the ipratropium bromide group and more frequently in the ipratropium bromide group than in the vehicle group. | ||||
1 Epistaxis reported by 7.0% of ipratropium bromide patients and 2.3% of vehicle patients, blood-tinged mucus by 2.0% of ipratropium bromide patients and 2.3% of vehicle patients. | ||||
2 Nasal irritation includes reports of nasal itching, nasal burning, nasal irritation and ulcerative rhinitis. | ||||
3 Other nasal symptoms include reports of nasal congestion, increased rhinorrhea, increased rhinitis, posterior nasal drip, sneezing, nasal polyps and nasal edema. | ||||
* All events are listed by their WHO term; rhinitis has been presented by descriptive terms for clarification. | ||||
| Headache | 9.8 | 0.6 | 9.2 | 0.0 |
| Upper respiratory | ||||
| tract infection | 9.8 | 1.4 | 7.2 | 1.4 |
| Epistaxis1 | 9.0 | 0.3 | 4.6 | 0.3 |
| Rhinitis* | ||||
| Nasal dryness | 5.1 | 0.0 | 0.9 | 0.3 |
| Nasal irritation2 | 2.0 | 0.0 | 1.7 | 0.6 |
| Other nasal symptoms3 | 3.1 | 1.1 | 1.7 | 0.3 |
| Pharyngitis | 8.1 | 0.3 | 4.6 | 0.0 |
| Nausea | 2.2 | 0.3 | 0.9 | 0.0 |
Ipratropium bromide nasal solution, 0.03% was well tolerated by most patients. The most frequently reported nasal adverse events were transient episodes of nasal dryness or epistaxis. These adverse events were mild or moderate in nature, none was considered serious, none resulted in hospitalization and most resolved spontaneously or following a dose reduction. Treatment for nasal dryness and epistaxis was required infrequently (2% or less) and consisted of local application of pressure or a moisturizing agent (e.g., petroleum jelly or saline nasal spray). Patient discontinuation for epistaxis or nasal dryness was infrequent in both the controlled (0.3% or less) and one-year, open-label (2% or less) trials. There was no evidence of nasal rebound (i.e., a clinically significant increase in rhinorrhea, posterior nasal drip, sneezing or nasal congestion severity compared to baseline) upon discontinuation of double-blind therapy in these trials.
Adverse events reported by less than 2% of the patients receiving ipratropium bromide nasal solution, 0.03% during the controlled clinical trials or during the open-label follow-up trial, which are potentially related to ipratropium bromide's local effects or systemic anticholinergic effects include: dry mouth/throat, dizziness, ocular irritation, blurred vision, conjunctivitis, hoarseness, cough, and taste perversion.
There were infrequent reports of skin rash in both the controlled and uncontrolled clinical studies.
No controlled clinical trials were conducted to investigate potential drug-drug interactions. There is potential for an additive interaction with other concomitantly administered medications with anticholinergic properties, including ipratropium bromide for oral inhalation.
The active ingredient in ipratropium bromide nasal solution is ipratropium bromide monohydrate. It is an anticholinergic agent chemically described as 8-azoniabicyclo (3.2.1) octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide, monohydrate (
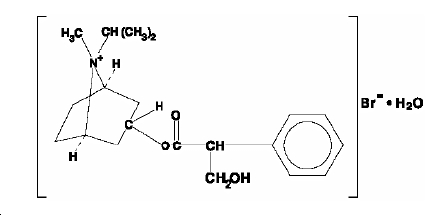
ipratropium bromide C20H30BrNO3•H2O
monohydrate Mol. Wt. 430.4
Ipratropium bromide is a white to off-white, crystalline substance. It is freely soluble in lower alcohols and water, existing in an ionized state in aqueous solutions, and relatively insoluble in non-polar media.
Ipratropium bromide nasal solution, 0.03% is a metered-dose, manual pump spray unit which delivers 21 mcg (70 mcL) ipratropium bromide per spray on an anhydrous basis in an isotonic, aqueous solution. It also contains the following inactive ingredients: benzalkonium chloride, edetate disodium, purified water and sodium chloride. Hydrochloric acid and/or sodium hydroxide may be added to adjust the pH to 4.5 to 4.9. Each bottle contains 345 sprays.