Irinotecan Hydrochloride Prescribing Information
- Early and late forms of diarrhea can occur. Early diarrhea may be accompanied by cholinergic symptoms which may be prevented or ameliorated by atropine. Late diarrhea can be life threatening and should be treated promptly with loperamide. Monitor patients with diarrhea and give fluid and electrolytes as needed. Institute antibiotic therapy if patients develop ileus, fever, or severe neutropenia. Interrupt irinotecan hydrochloride injection and reduce subsequent doses if severe diarrhea occurs[see.and
2.1 Colorectal Single Agent Regimens 1 and 2Administer irinotecan hydrochloride injection as a 90-minute intravenous infusion. The currently recommended regimens are shown in Table 1.
A reduction in the starting dose by one dose level of irinotecan hydrochloride injection may be considered for patients with any of the following conditions: prior pelvic/abdominal radiotherapy, performance status of 2, or increased bilirubin levels. Dosing for patients with bilirubin >2 mg/dL cannot be recommended because there is insufficient information to recommend a dose in these patients.
Table 1. Single-Agent Regimens of Irinotecan Hydrochloride Injection and Dose Modifications Regimen 1(weekly)Subsequent doses may be adjusted as high as 150 mg/m2or to as low as 50 mg/m2in 25 to 50 mg/m2decrements depending upon individual patient tolerance.
125 mg/m2intravenous infusion over 90 minutes, days 1,8,15,22 then 2-week rest Starting Dose and Modified Dose LevelsProvided intolerable toxicity does not develop, treatment with additional cycles may be continued indefinitely as long as patients continue to experience clinical benefit.
(mg/m2)Starting Dose Dose Level -1 Dose Level -2 125 100 75 Regimen 2
(every 3 weeks)Subsequent doses may be adjusted as low as 200 mg/m2in 50 mg/m2decrements depending upon individual patient tolerance.350 mg/m2intravenous infusion over 90 minutes, once every 3 weeks Starting Dose and Modified Dose Levels (mg/m2)Starting Dose Dose Level -1 Dose Level -2 350 300 250 Dose ModificationsBased on recommended dose-levels described in Table 1, Single-Agent Regimens of Irinotecan Hydrochloride Injection and Dose Modifications, subsequent doses should be adjusted as suggested in Table 2, Recommended Dose Modifications for Single-Agent Schedules. All dose modifications should be based on the worst preceding toxicity.
Table 2. Recommended Dose Modifications For Single-Agent SchedulesAll dose modifications should be based on the worst preceding toxicity A new cycle of therapy should not begin until the granulocyte count has recovered to ≥1500/mm3, and the platelet count has recovered to ≥100,000/mm3, and treatment-related diarrhea is fully resolved. Treatment should be delayed 1 to 2 weeks to allow for recovery from treatment-related toxicities. If the patient has not recovered after a 2-week delay, consideration should be given to discontinuing irinotecan hydrochloride injection.Worst Toxicity
NCI GradeNational Cancer Institute Common Toxicity Criteria (version 1.0)
(Value)During a Cycle of TherapyAt the Start of the Next Cycles of Therapy (After Adequate Recovery), Compared with the Starting Dose in the Previous CycleWeeklyWeeklyOnce Every 3 WeeksNo toxicity Maintain dose level ↑ 25 mg/m2up to a maximum dose of 150 mg/m2 Maintain dose level Neutropenia1 (1500 to 1999/mm3) Maintain dose level Maintain dose level Maintain dose level 2 (1000 to 1499/mm3) ↓ 25 mg/m2 Maintain dose level Maintain dose level 3 (500 to 999/mm3) Omit dose until resolved to ≤ grade 2, then ↓ 25 mg/m2 ↓ 25 mg/m2 ↓ 50 mg/m2 4 (<500/mm3) Omit dose until resolved to ≤ grade 2, then ↓ 50 mg/m2 ↓ 50 mg/m2 ↓ 50 mg/m2 Neutropenic feverOmit dose until resolved, then ↓ 50 mg/m2when resolved ↓ 50 mg/m2 ↓ 50 mg/m2 Other hematologic toxicitiesDose modifications for leukopenia, thrombocytopenia, and anemia during a cycle of therapy and at the start of subsequent cycles of therapy are also based on NCI toxicity criteria and are the same as recommended for neutropenia above. Diarrhea1 (2–3 stools/day > pretxPretreatment) Maintain dose level Maintain dose level Maintain dose level 2 (4–6 stools/day > pretx) ↓ 25 mg/m2 Maintain dose level Maintain dose level 3 (7–9 stools/day > pretx) Omit dose until resolved to ≤ grade 2, then ↓ 25 mg/m2 ↓ 25 mg/m2 ↓ 50 mg/m2 4 (≥10 stools/day > pretx) Omit dose until resolved to ≤ grade 2 then ↓ 50 mg/m2 ↓ 50 mg/m2 ↓ 50 mg/m2 Other nonhematologicExcludes alopecia, anorexia, asthenia
toxicities1 Maintain dose level Maintain dose level Maintain dose level 2 ↓ 25 mg/m2 ↓ 25 mg/m2 ↓ 50 mg/m2 3 Omit dose until resolved to ≤ grade 2, then ↓ 25 mg/m2 ↓ 25 mg/m2 ↓ 50 mg/m2 4 Omit dose until resolved to ≤ grade 2, then ↓ 50 mg/m2 ↓ 50 mg/m2 ↓ 50 mg/m2 ]5.1 Diarrhea and Cholinergic ReactionsEarly diarrhea (occurring during or shortly after infusion of irinotecan hydrochloride injection) is usually transient and infrequently severe. It may be accompanied by cholinergic symptoms of rhinitis, increased salivation, miosis, lacrimation, diaphoresis, flushing, and intestinal hyperperistalsis that can cause abdominal cramping. Bradycardia may also occur. Early diarrhea and other cholinergic symptoms may be prevented or treated. Consider prophylactic or therapeutic administration of 0.25 mg to 1 mg of intravenous or subcutaneous atropine (unless clinically contraindicated). These symptoms are expected to occur more frequently with higher irinotecan doses.
Late diarrhea (generally occurring more than 24 hours after administration of irinotecan hydrochloride injection) can be life threatening since it may be prolonged and may lead to dehydration, electrolyte imbalance, or sepsis. Grade 3–4 late diarrhea occurred in 23–31% of patients receiving weekly dosing. In the clinical studies, the median time to the onset of late diarrhea was 5 days with 3-week dosing and 11 days with weekly dosing. Late diarrhea can be complicated by colitis, ulceration, bleeding, ileus, obstruction, and infection. Cases of megacolon and intestinal perforation have been reported. Patients should have loperamide readily available to begin treatment for late diarrhea. Begin loperamide at the first episode of poorly formed or loose stools or the earliest onset of bowel movements more frequent than normal. One dosage regimen for loperamide is 4 mg at the first onset of late diarrhea and then 2 mg every 2 hours until the patient is diarrhea-free for at least 12 hours. Loperamide is not recommended to be used for more than 48 consecutive hours at these doses, because of the risk of paralytic ileus. During the night, the patient may take 4 mg of loperamide every 4 hours. Monitor and replace fluid and electrolytes. Use antibiotic support for ileus, fever, or severe neutropenia. Subsequent weekly chemotherapy treatments should be delayed in patients until return of pretreatment bowel function for at least 24 hours without anti-diarrhea medication. Patients must not be treated with irinotecan until resolution of the bowel obstruction. If grade 2, 3, or 4 late diarrhea recurs, subsequent doses of irinotecan hydrochloride injection should be decreased [
see Dosage and Administration (2)].Avoid diuretics or laxatives in patients with diarrhea.
- Severe myelosuppression may occur[see.]
5.2 MyelosuppressionIrinotecan Hydrochloride Injection can cause severe myelosuppression. Bacterial, viral, and fungal infections have occurred in patients treated with Irinotecan Hydrochloride Injection.
Deaths due to sepsis following severe neutropenia have been reported in patients treated with irinotecan hydrochloride injection. In the clinical studies evaluating the weekly dosage schedule, neutropenic fever (concurrent NCI grade 4 neutropenia and fever of grade 2 or greater) occurred in 3% of the patients; 6% of patients received G-CSF for the treatment of neutropenia. Manage febrile neutropenia promptly with antibiotic support [
seeWarnings and Precautions (5.1)]. Hold irinotecan hydrochloride injection if neutropenic fever occurs or if the absolute neutrophil count drops <1000/mm3. After recovery to an absolute neutrophil count ≥1000/mm3, subsequent doses of irinotecan hydrochloride injection should be reduced [see Dosage and Administration (2)].When evaluated in the trials of weekly administration, the frequency of grade 3 and 4 neutropenia was higher in patients who received previous pelvic/abdominal irradiation than in those who had not received such irradiation (48% [13/27] versus 24% [67/277]; p=0.04). Patients who have previously received pelvic/abdominal irradiation are at increased risk of severe myelosuppression following the administration of irinotecan hydrochloride injection. Based on sparse available data, the concurrent administration of irinotecan hydrochloride injection with irradiation is not recommended.
Patients with baseline serum total bilirubin levels of 1.0 mg/dL or more also had a greater likelihood of experiencing first-cycle grade 3 or 4 neutropenia than those with bilirubin levels that were less than 1.0 mg/dL (50% [19/38] versus 18% [47/266]; p<0.001). Patients with deficient glucuronidation of bilirubin, such as those with Gilbert's syndrome, may be at greater risk of myelosuppression when receiving therapy with irinotecan hydrochloride injection.
Irinotecan Hydrochloride Injection, USP is indicated for patients with metastatic carcinoma of the colon or rectum whose disease has recurred or progressed following initial fluorouracil-based therapy.
Irinotecan hydrochloride injection is available in two single-dose sizes:
- 2 mL-fill vial containing 40 mg irinotecan hydrochloride
- 5 mL-fill vial containing 100 mg irinotecan hydrochloride
Verify the pregnancy status in female patients of reproductive potential prior to initiating irinotecan hydrochloride injection.
Irinotecan hydrochloride injection can cause fetal harm when administered to a pregnant woman.
Advise female patients of reproductive potential to use effective contraception during treatment and for 6 months after the final dose of irinotecan hydrochloride injection
Based on findings from animal studies and its mechanism of action, irinotecan hydrochloride injection can cause fetal harm when administered to a pregnant woman
Available postmarketing and published data reporting the use of irinotecan hydrochloride injection in pregnant women, are insufficient and confounded by the concomitant use of other cytotoxic drugs, to evaluate for any drug-associated risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal studies, intravenous administration of irinotecan to rats and rabbits during the period of organogenesis resulted in embryofetal mortality and teratogenicity in pregnant animals at exposures lower than the human exposure based on AUC at the clinical dose of 125 mg/m2
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Radioactivity related to 14C-irinotecan crosses the placenta of rats following intravenous administration. Intravenous administration of irinotecan to rats at a dose of 6 mg/kg/day (approximately 0.2 times the clinical exposure (AUC) at the 125 mg/m2dose based on exposure data from a separate rat study) during the period of organogenesis resulted in increased post-implantation loss and decreased numbers of live fetuses; at doses ≥ 1.2 mg/kg/day (approximately 0.03 times the clinical exposure (AUC) at the 125 mg/m2 dose based on exposure data from a separate rat study) there were increases in a variety of external, visceral, and skeletal abnormalities. Administration of irinotecan to pregnant rabbits at a dose of 6 mg/kg (approximately half of the clinical dose of 125 mg/m2 based on BSA) resulted in similar findings to those in rats, with increased post-implantation loss, decreased live fetuses, and increased external, visceral, and skeletal abnormalities.
Irinotecan administered to rat dams for the period following organogenesis through weaning at doses of 6 mg/kg/day caused decreased learning ability and decreased female body weights in the offspring.
Long-term carcinogenicity studies with irinotecan were not conducted. Rats were, however, administered intravenous doses of 2 mg/kg or 25 mg/kg irinotecan once per week for 13 weeks (in separate studies, the 25 mg/kg dose produced an irinotecan Cmaxand AUC that were about 7.0 times and 1.3 times the respective values in patients administered 125 mg/m2weekly) and were then allowed to recover for 91 weeks. Under these conditions, there was a significant linear trend with dose for the incidence of combined uterine horn endometrial stromal polyps and endometrial stromal sarcomas. Irinotecan was clastogenic both
No significant adverse effects on fertility and general reproductive performance were observed after intravenous administration of irinotecan in doses of up to 6 mg/kg/day to rats and rabbits; however, atrophy of male reproductive organs was observed after multiple daily irinotecan doses both in rodents at 20 mg/kg and in dogs at 0.4 mg/kg. In separate studies in rodents, this dose produced an irinotecan Cmaxand AUC about 5 and 1 times, respectively, of the corresponding values in patients administered 125 mg/m2weekly. In dogs this dose produced an irinotecan Cmaxand AUC about one-half and 1/15th, respectively, of the corresponding values in patients administered 125 mg/m2weekly.
Due to the potential for genotoxicity, advise male patients with female partners of reproductive potential to use condoms during treatment and for 3 months after the final dose of irinotecan hydrochloride injection
Long-term carcinogenicity studies with irinotecan were not conducted. Rats were, however, administered intravenous doses of 2 mg/kg or 25 mg/kg irinotecan once per week for 13 weeks (in separate studies, the 25 mg/kg dose produced an irinotecan Cmaxand AUC that were about 7.0 times and 1.3 times the respective values in patients administered 125 mg/m2weekly) and were then allowed to recover for 91 weeks. Under these conditions, there was a significant linear trend with dose for the incidence of combined uterine horn endometrial stromal polyps and endometrial stromal sarcomas. Irinotecan was clastogenic both
No significant adverse effects on fertility and general reproductive performance were observed after intravenous administration of irinotecan in doses of up to 6 mg/kg/day to rats and rabbits; however, atrophy of male reproductive organs was observed after multiple daily irinotecan doses both in rodents at 20 mg/kg and in dogs at 0.4 mg/kg. In separate studies in rodents, this dose produced an irinotecan Cmaxand AUC about 5 and 1 times, respectively, of the corresponding values in patients administered 125 mg/m2weekly. In dogs this dose produced an irinotecan Cmaxand AUC about one-half and 1/15th, respectively, of the corresponding values in patients administered 125 mg/m2weekly.
Based on postmarketing reports, female fertility may be impaired by treatment with irinotecan hydrochloride injection. Menstrual dysfunction has been reported following irinotecan hydrochloride injection administration.
Based on findings from animal studies, male fertility may be impaired by treatment with irinotecan hydrochloride injection
Long-term carcinogenicity studies with irinotecan were not conducted. Rats were, however, administered intravenous doses of 2 mg/kg or 25 mg/kg irinotecan once per week for 13 weeks (in separate studies, the 25 mg/kg dose produced an irinotecan Cmaxand AUC that were about 7.0 times and 1.3 times the respective values in patients administered 125 mg/m2weekly) and were then allowed to recover for 91 weeks. Under these conditions, there was a significant linear trend with dose for the incidence of combined uterine horn endometrial stromal polyps and endometrial stromal sarcomas. Irinotecan was clastogenic both
No significant adverse effects on fertility and general reproductive performance were observed after intravenous administration of irinotecan in doses of up to 6 mg/kg/day to rats and rabbits; however, atrophy of male reproductive organs was observed after multiple daily irinotecan doses both in rodents at 20 mg/kg and in dogs at 0.4 mg/kg. In separate studies in rodents, this dose produced an irinotecan Cmaxand AUC about 5 and 1 times, respectively, of the corresponding values in patients administered 125 mg/m2weekly. In dogs this dose produced an irinotecan Cmaxand AUC about one-half and 1/15th, respectively, of the corresponding values in patients administered 125 mg/m2weekly.
Long-term carcinogenicity studies with irinotecan were not conducted. Rats were, however, administered intravenous doses of 2 mg/kg or 25 mg/kg irinotecan once per week for 13 weeks (in separate studies, the 25 mg/kg dose produced an irinotecan Cmaxand AUC that were about 7.0 times and 1.3 times the respective values in patients administered 125 mg/m2weekly) and were then allowed to recover for 91 weeks. Under these conditions, there was a significant linear trend with dose for the incidence of combined uterine horn endometrial stromal polyps and endometrial stromal sarcomas. Irinotecan was clastogenic both
No significant adverse effects on fertility and general reproductive performance were observed after intravenous administration of irinotecan in doses of up to 6 mg/kg/day to rats and rabbits; however, atrophy of male reproductive organs was observed after multiple daily irinotecan doses both in rodents at 20 mg/kg and in dogs at 0.4 mg/kg. In separate studies in rodents, this dose produced an irinotecan Cmaxand AUC about 5 and 1 times, respectively, of the corresponding values in patients administered 125 mg/m2weekly. In dogs this dose produced an irinotecan Cmaxand AUC about one-half and 1/15th, respectively, of the corresponding values in patients administered 125 mg/m2weekly.
Long-term carcinogenicity studies with irinotecan were not conducted. Rats were, however, administered intravenous doses of 2 mg/kg or 25 mg/kg irinotecan once per week for 13 weeks (in separate studies, the 25 mg/kg dose produced an irinotecan Cmaxand AUC that were about 7.0 times and 1.3 times the respective values in patients administered 125 mg/m2weekly) and were then allowed to recover for 91 weeks. Under these conditions, there was a significant linear trend with dose for the incidence of combined uterine horn endometrial stromal polyps and endometrial stromal sarcomas. Irinotecan was clastogenic both
No significant adverse effects on fertility and general reproductive performance were observed after intravenous administration of irinotecan in doses of up to 6 mg/kg/day to rats and rabbits; however, atrophy of male reproductive organs was observed after multiple daily irinotecan doses both in rodents at 20 mg/kg and in dogs at 0.4 mg/kg. In separate studies in rodents, this dose produced an irinotecan Cmaxand AUC about 5 and 1 times, respectively, of the corresponding values in patients administered 125 mg/m2weekly. In dogs this dose produced an irinotecan Cmaxand AUC about one-half and 1/15th, respectively, of the corresponding values in patients administered 125 mg/m2weekly.
Long-term carcinogenicity studies with irinotecan were not conducted. Rats were, however, administered intravenous doses of 2 mg/kg or 25 mg/kg irinotecan once per week for 13 weeks (in separate studies, the 25 mg/kg dose produced an irinotecan Cmaxand AUC that were about 7.0 times and 1.3 times the respective values in patients administered 125 mg/m2weekly) and were then allowed to recover for 91 weeks. Under these conditions, there was a significant linear trend with dose for the incidence of combined uterine horn endometrial stromal polyps and endometrial stromal sarcomas. Irinotecan was clastogenic both
No significant adverse effects on fertility and general reproductive performance were observed after intravenous administration of irinotecan in doses of up to 6 mg/kg/day to rats and rabbits; however, atrophy of male reproductive organs was observed after multiple daily irinotecan doses both in rodents at 20 mg/kg and in dogs at 0.4 mg/kg. In separate studies in rodents, this dose produced an irinotecan Cmaxand AUC about 5 and 1 times, respectively, of the corresponding values in patients administered 125 mg/m2weekly. In dogs this dose produced an irinotecan Cmaxand AUC about one-half and 1/15th, respectively, of the corresponding values in patients administered 125 mg/m2weekly.
Irinotecan hydrochloride injection is contraindicated in patients with a known hypersensitivity to the drug or its excipients.
Irinotecan Hydrochloride Injection, USP is an antineoplastic agent of the topoisomerase I inhibitor class.
Irinotecan Hydrochloride Injection, USP is supplied as a sterile, pale yellow, clear, aqueous solution. Each milliliter of solution contains 20 mg of irinotecan hydrochloride (on the basis of the trihydrate salt), 45 mg of sorbitol, NF, and 0.9 mg of lactic acid, USP. The pH of the solution has been adjusted to 3.5 (range, 3.0 to 3.8) with sodium hydroxide or hydrochloric acid. Irinotecan Hydrochloride Injection, USP is intended for dilution with 5% Dextrose Injection, USP (D5W), or 0.9% Sodium Chloride Injection, USP, prior to intravenous infusion. The preferred diluent is 5% Dextrose Injection, USP.
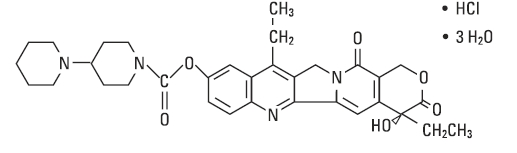
Irinotecan hydrochloride is a semisynthetic derivative of camptothecin, an alkaloid extract from plants such as
The chemical name is