Isoflurane
Isoflurane Prescribing Information
Isoflurane USP may be used for induction and maintenance of general anesthesia. Adequate data have not been developed to establish its application in obstetrical anesthesia.
Isoflurane is a clear, colorless, stable liquid containing no additives or chemical stabilizers, 100% isoflurane.
In the event of overdosage, or what may appear to be overdosage, the following action should be taken, as appropriate:
Stop drug administration, establish a clear airway, and initiate assisted or controlled ventilation with pure oxygen. Monitor cardiovascular function and manage signs of poor end-organ perfusion as clinically indicated.
Isoflurane USP is contraindicated in patients:
• in whom general anesthesia is contraindicated.
• with known sensitivity to Isoflurane USP or to other halogenated agents
Cases of mild, moderate and severe postoperative hepatic dysfunction or hepatitis with or without jaundice, including fatal hepatic necrosis and hepatic failure, have been reported with isoflurane.
Such reactions can represent hypersensitivity hepatitis, a known risk of exposure to halogenated anesthetics, including isoflurane. As with other halogenated anesthetic agents, Isoflurane USP may cause sensitivity hepatitis in patients who have been sensitized by previous exposure to halogenated anesthetics
Clinical judgment should be exercised when isoflurane is used in patients with underlying hepatic conditions or under treatment with drugs known to cause hepatic dysfunction.
As with all halogenated anesthetics, repeated anesthetics within a short period of time may result in increased effects, particularly in patients with underlying hepatic conditions, or additive effects in patients treated with drugs known to cause hepatic dysfunction. Evaluate the need for repeated exposure in each individual patient and adjust the dose of isoflurane based on signs and symptoms of adequate depth of anesthesia if repeated exposure in a short period of time is clinically indicated.
• with known or suspected genetic susceptibility to malignant hyperthermia
• with a history of confirmed hepatitis due to a halogenated inhalational anesthetic or a history of unexplained moderate to severe hepatic dysfunction (e.g., jaundice associated with fever and/or eosinophilia) after anesthesia with isoflurane or other halogenated inhalational anesthetics.
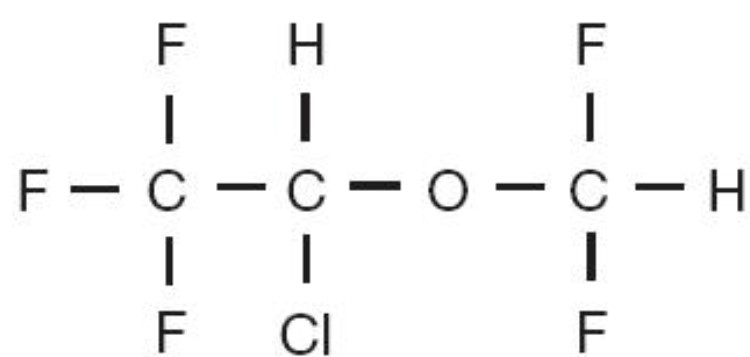
Isoflurane USP (isoflurane, USP), a nonflammable liquid administered by vaporizing, is a general inhalation anesthetic drug. It is 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether, and its structural formula is:
| Molecular weight | 184.5 | |
| Boiling point at 760 mm Hg | 48.5°C | |
Refractive index n 20/D | 1.2990-1.3005 | |
| Specific gravity 25°/25°C | 1.496 | |
| Vapor pressure in mm Hg** | 20°C | 238 |
| 25°C | 295 | |
| 30°C | 367 | |
| 35°C | 450 | |
**Equation for vapor pressure calculation: log | A = 8.056 B=−1664.58 T = °C + 273.16 (Kelvin) | |
Partition coefficients at 37°C | ||
| Water/gas | 0.61 | |
| Blood/gas | 1.43 | |
| Oil/gas | 90.8 | |
Partition coefficients at 25°C – rubber and plastic | ||
| Conductive rubber/gas | 62.0 | |
| Butyl rubber/gas | 75.0 | |
| Polyvinyl chloride/gas | 110.0 | |
| Polyethylene/gas | ~2.0 | |
| Polyurethane/gas | ~1.4 | |
| Polyolefin/gas | ~1.1 | |
| Butyl acetate/gas | ~2.5 | |
| Purity by gas chromatography | >99.9% | |
| Lower limit of flammability in oxygen or nitrous oxide at 9 joules/sec. and 23°C | None | |
| Lower limit of flammability in oxygen or nitrous oxide at 900 joules/sec. and 23°C | Greater than useful concentration in anesthesia. |
Isoflurane is a clear, colorless, stable liquid containing no additives or chemical stabilizers. Isoflurane has a mildly pungent, musty, ethereal odor. Samples stored in indirect sunlight in clear, colorless glass for five years, as well as samples directly exposed for 30 hours to a 2 amp, 115 volt, 60 cycle long wave U.V. light were unchanged in composition as determined by gas chromatography. Isoflurane in one normal sodium methoxide-methanol solution, a strong base, for over six months consumed essentially no alkali, indicative of strong base stability. Isoflurane does not decompose in the presence of soda lime (at normal operating temperatures), and does not attack aluminum, tin, brass, iron or copper.
Induction of and recovery from isoflurane anesthesia are rapid. Isoflurane has a mild pungency which limits the rate of induction, although excessive salivation or tracheobronchial secretions do not appear to be stimulated. Pharyngeal and laryngeal reflexes are readily obtunded. The level of anesthesia may be changed rapidly with isoflurane. Isoflurane is a profound respiratory depressant. As anesthetic dose is increased, tidal volume decreases and respiratory rate is unchanged. This depression is partially reversed by surgical stimulation, even at deeper levels of anesthesia. Isoflurane evokes a sigh response reminiscent of that seen with diethyl ether and enflurane, although the frequency is less than with enflurane.
Blood pressure decreases with induction of anesthesia but returns toward normal with surgical stimulation. Progressive increases in depth of anesthesia produce corresponding decreases in blood pressure. Nitrous oxide diminishes the inspiratory concentration of isoflurane required to reach a desired level of anesthesia and may reduce the arterial hypotension seen with isoflurane alone. Heart rhythm is remarkably stable. With controlled ventilation and normal PaCO
2, cardiac output is maintained despite increasing depth of anesthesia, primarily through an increase in heart rate which compensates for a reduction in stroke volume. The hypercapnia which attends spontaneous ventilation during isoflurane anesthesia further increases heart rate and raises cardiac output above awake levels.
Muscle relaxation is often adequate for intra-abdominal operations at normal levels of anesthesia. Complete muscle paralysis can be attained with small doses of neuromuscular blocking agents. ALL COMMONLY USED NEUROMUSCULAR BLOCKING AGENTS ARE MARKEDLY POTENTIATED WITH ISOFLURANE, THE EFFECT BEING MOST PROFOUND WITH THE NONDEPOLARIZING TYPE. Neostigmine reverses the effect of nondepolarizing neuromuscular blocking agents in the presence of isoflurane. All commonly used neuromuscular blocking agents are compatible with isoflurane.
Isoflurane can produce coronary vasodilation at the arteriolar level in selected animal models; the drug is probably also a coronary dilator in humans. Isoflurane, like some other coronary arteriolar dilators, has been shown to divert blood from collateral dependent myocardium to normally perfused areas in an animal model (“coronary steal”). Clinical trials to date evaluating myocardial ischemia, infarction and death as outcome parameters have not established that the coronary arteriolar dilation property of isoflurane is associated with coronary steal or myocardial ischemia in patients with coronary artery disease.