Isoproterenol Hydrochloride
Isoproterenol Hydrochloride Prescribing Information
Isoproterenol hydrochloride injection is indicated:
- For mild or transient episodes of heart block that do not require electric shock or pacemaker therapy.
- For serious episodes of heart block and Adams-Stokes attacks (except when caused by ventricular tachycardia or fibrillation). (See CONTRAINDICATIONS.)
- For use in cardiac arrest until electric shock or pacemaker therapy, the treatments of choice, is available. (See CONTRAINDICATIONS.)
- For bronchospasm occurring during anesthesia.
- As an adjunct to fluid and electrolyte replacement therapy and the use of other drugs and procedures in the treatment of hypovolemic and septic shock, low cardiac output (hypoperfusion) states, congestive heart failure, and cardiogenic shock. (See WARNINGS.)
Start isoproterenol hydrochloride injection at the lowest recommended dose and increase the rate of administration gradually if necessary while carefully monitoring the patient. The usual route of administration is by intravenous infusion or bolus intravenous injection. In dire emergencies, the drug may be administered by intracardiac injection. If time is not of the utmost importance, initial therapy by intramuscular or subcutaneous injection is preferred.
Route of Administration | Preparation of Dilution | Initial Dose | Subsequent Dose Range* |
| Bolus intravenous injection | Dilute 1 mL (0.2 mg) in 9 mL of Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP | 0.02 mg to 0.06 mg (1 mL to 3 mL of diluted solution) | 0.01 mg to 0.2 mg (0.5 mL to 10 mL of diluted solution) |
| Intravenous infusion | Dilute 10 mL (2 mg) in 500 mL of 5% Dextrose Injection, USP | 5 mcg/min. (1.25 mL of diluted solution per minute) | |
| Intramuscular | Use Solution undiluted | 0.2 mg (1 mL) | 0.02 mg to 1 mg (0.1 mL to 5 mL) |
| Subcutaneous | Use Solution undiluted | 0.2 mg (1 mL) | 0.15 mg to 0.2 mg (0.75 mL to 1 mL) |
| Intracardiac | Use Solution undiluted | 0.02 mg (0.1 mL) |
* Subsequent dosage and method of administration depend on the ventricular rate and the rapidity with which the cardiac pacemaker can take over when the drug is gradually withdrawn.
There are no well-controlled studies in children to establish appropriate dosing; however, the American Heart Association recommends an initial infusion rate of 0.1 mcg/kg/min, with the usual range being 0.1 mcg/kg/min to 1 mcg/kg/min.
Route of Administration | Preparation of Dilution † | Infusion Rate †† |
| Intravenous infusion | Dilute 5 mL (1 mg) in 500 mL of 5% Dextrose Injection, USP | 0.5 mcg to 5 mcg per minute(0.25 mL to 2.5 mL of diluted solution) |
† Concentrations up to 10 times greater have been used when limitation of volume is essential.
†† Rates over 30 mcg per minute have been used in advanced stages of shock. The rate of infusion should be adjusted on the basis of heart rate, central venous pressure, systemic blood pressure, and urine flow. If the heart rate exceeds 110 beats per minute, it may be advisable to decrease or temporarily discontinue the infusion.
Route of Administration | Preparation of Dilution | Initial Dose | Subsequent Dose |
| Bolus intravenous injection | Dilute 1 mL (0.2 mg) in 9 mL of Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP | 0.01 mg to 0.02 mg (0.5 mL to 1 mL of diluted solution) | The initial dose may be repeated when necessary |
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Such solution should not be used.
Use of isoproterenol hydrochloride injection is contraindicated in patients with tachyarrhythmias; tachycardia or heart block caused by digitalis intoxication; ventricular arrhythmias which require inotropic therapy; and angina pectoris.
The following reactions to isoproterenol hydrochloride injection have been reported:
In a few patients, presumably with organic disease of the AV node and its branches, isoproterenol hydrochloride injection has been reported to precipitate Adams-Stokes seizures during normal sinus rhythm or transient heart block.
Isoproterenol hydrochloride injection and epinephrine should not be administered simultaneously because both drugs are direct cardiac stimulants and their combined effects may induce serious arrhythmias. The drugs may, however, be administered alternately provided a proper interval has elapsed between doses.
Avoid isoproterenol hydrochloride injection when potent inhalational anesthetics such as halothane are employed because of potential to sensitize the myocardium to effects of sympathomimetic amines.
Isoproterenol hydrochloride is 3,4-dihydroxy--[(isopropylamino)methyl] benzyl alcohol hydrochloride, a synthetic sympathomimetic amine that is structurally related to epinephrine but acts almost exclusively on beta receptors. The molecular formula is C11H17NO3 HCl. It has a molecular weight of 247.72 and the following structural formula:
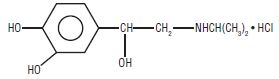
Isoproterenol hydrochloride is a racemic compound.
Each milliliter of the sterile solution contains:
Isoproterenol hydrochloride injection, USP 0.2 mg
Edetate Disodium (EDTA) 0.2 mg
Sodium Chloride 7.0 mg
Sodium Citrate, Dihydrate 2.07 mg
Citric Acid, Anhydrous 2.5 mg
Water for Injection 1.0 mL
The pH is adjusted between 2.5 and 4.5 with hydrochloric acid or sodium hydroxide.
The sterile solution is nonpyrogenic and can be administered by the intravenous, intramuscular, subcutaneous, or intracardiac routes.