Ivermectin
Ivermectin Prescribing Information
Ivermectin tablets are indicated for the treatment of the following infections:
Ivermectin tablet is indicated for the treatment of intestinal (i.e., nondisseminated) strongyloidiasis due to the nematode parasite
This indication is based on clinical studies of both comparative and open-label designs, in which 64-100% of infected patients were cured following a single 200-mcg/kg dose of ivermectin. (See
Following oral administration of ivermectin, plasma concentrations are approximately proportional to the dose. In two studies, after single 12-mg doses of ivermectin tablets in fasting healthy volunteers (representing a mean dose of 165 mcg/kg), the mean peak plasma concentrations of the major component (H2B1a) were 46.6 (± 21.9) (range: 16.4-101.1) and 30.6 (± 15.6) (range: 13.9-68.4) ng/mL, respectively, at approximately 4 hours after dosing. Ivermectin is metabolized in the liver, and ivermectin and/or its metabolites are excreted almost exclusively in the feces over an estimated 12 days, with less than 1% of the administered dose excreted in the urine. The plasma half-life of ivermectin in man is approximately 18 hours following oral administration.
The safety and pharmacokinetic properties of ivermectin were further assessed in a multiple-dose clinical pharmacokinetic study involving healthy volunteers. Subjects received oral doses of 30 to 120 mg (333 to 2000 mcg/kg) ivermectin in a fasted state or 30 mg (333 to 600 mcg/kg) ivermectin following a standard high-fat (48.6 g of fat) meal. Administration of 30 mg ivermectin following a high-fat meal resulted in an approximate 2.5-fold increase in bioavailability relative to administration of 30 mg ivermectin in the fasted state.
Ivermectin is a member of the avermectin class of broad-spectrum antiparasitic agents which have a unique mode of action. Compounds of the class bind selectively and with high affinity to glutamate- gated chloride ion channels which occur in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
The selective activity of compounds of this class is attributable to the facts that some mammals do not have glutamate-gated chloride channels and that the avermectins have a low affinity for mammalian ligand-gated chloride channels. In addition, ivermectin does not readily cross the blood-brain barrier in humans.
Ivermectin is active against various life-cycle stages of many but not all nematodes. It is active against the tissue microfilariae of
Two controlled clinical studies using albendazole as the comparative agent were carried out in international sites where albendazole is approved for the treatment of strongyloidiasis of the gastrointestinal tract, and three controlled studies were carried out in the U.S. and internationally using thiabendazole as the comparative agent. Efficacy, as measured by cure rate, was defined as the absence of larvae in at least two follow-up stool examinations 3 to 4 weeks post-therapy. Based on this criterion, efficacy was significantly greater for ivermectin tablets (a single dose of 170 to 200 mcg/kg) than for albendazole (200 mg b.i.d. for 3 days). Ivermectin tablets administered as a single dose of 200 mcg/kg for 1 day was as efficacious as thiabendazole administered at 25 mg/kg b.i.d. for 3 days.
Summary of Cure Rates for Ivermectin Versus Comparative Agents in the Treatment of Strongyloidiasis
| Cure Rate*(%) | ||
| Ivermectin** | Comparative Agent | |
| Albendazole***Comparative | ||
| International Study | 24/26 (92) | 12/22 (55) |
| WHO Study | 126/152 (83) | 67/149 (45) |
| Thiabendazole†Comparative | ||
| International Study | 9/14 (64) | 13/15 (87) |
| US Studies | 14/14 (100) | 16/17 (94) |
* Number and % of evaluable patients
** 170-200 mcg/kg
*** 200 mg b.i.d. for 3 days
†25 mg/kg b.i.d. for 3 days
In one study conducted in France, a non-endemic area where there was no possibility of reinfection, several patients were observed to have recrudescence of
The evaluation of ivermectin tablets in the treatment of onchocerciasis is based on the results of clinical studies involving 1278 patients. In a double-blind, placebo-controlled study involving adult patients with moderate to severe onchocercal infection, patients who received a single dose of 150 mcg/kg ivermectin tablets experienced an 83.2% and 99.5% decrease in skin microfilariae count (geometric mean) 3 days and 3 months after the dose, respectively. A marked reduction of >90% was maintained for up to 12 months after the single dose. As with other microfilaricidal drugs, there was an increase in the microfilariae count in the anterior chamber of the eye at day 3 after treatment in some patients. However, at 3 and 6 months after the dose, a significantly greater percentage of patients treated with ivermectin tablets had decreases in microfilariae count in the anterior chamber than patients treated with placebo.
In a separate open study involving pediatric patients ages 6 to 13 (n=103; weight range: 17-41 kg), similar decreases in skin microfilariae counts were observed for up to 12 months after dosing.
Ivermectin tablet is indicated for the treatment of onchocerciasis due to the nematode parasite
This indication is based on randomized, double-blind, placebo-controlled and comparative studies conducted in 1427 patients in onchocerciasis-endemic areas of West Africa. The comparative studies used diethylcarbamazine citrate (DEC-C).
NOTE: Ivermectin tablet has no activity against adult
The recommended dosage of ivermectin tablets for the treatment of strongyloidiasis is a single oral dose designed to provide approximately 200 mcg of ivermectin per kg of body weight. See Table 1 for dosage guidelines. Patients should take tablets on an empty stomach with water. (See
Following oral administration of ivermectin, plasma concentrations are approximately proportional to the dose. In two studies, after single 12-mg doses of ivermectin tablets in fasting healthy volunteers (representing a mean dose of 165 mcg/kg), the mean peak plasma concentrations of the major component (H2B1a) were 46.6 (± 21.9) (range: 16.4-101.1) and 30.6 (± 15.6) (range: 13.9-68.4) ng/mL, respectively, at approximately 4 hours after dosing. Ivermectin is metabolized in the liver, and ivermectin and/or its metabolites are excreted almost exclusively in the feces over an estimated 12 days, with less than 1% of the administered dose excreted in the urine. The plasma half-life of ivermectin in man is approximately 18 hours following oral administration.
The safety and pharmacokinetic properties of ivermectin were further assessed in a multiple-dose clinical pharmacokinetic study involving healthy volunteers. Subjects received oral doses of 30 to 120 mg (333 to 2000 mcg/kg) ivermectin in a fasted state or 30 mg (333 to 600 mcg/kg) ivermectin following a standard high-fat (48.6 g of fat) meal. Administration of 30 mg ivermectin following a high-fat meal resulted in an approximate 2.5-fold increase in bioavailability relative to administration of 30 mg ivermectin in the fasted state.
Ivermectin is a member of the avermectin class of broad-spectrum antiparasitic agents which have a unique mode of action. Compounds of the class bind selectively and with high affinity to glutamate- gated chloride ion channels which occur in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
The selective activity of compounds of this class is attributable to the facts that some mammals do not have glutamate-gated chloride channels and that the avermectins have a low affinity for mammalian ligand-gated chloride channels. In addition, ivermectin does not readily cross the blood-brain barrier in humans.
Ivermectin is active against various life-cycle stages of many but not all nematodes. It is active against the tissue microfilariae of
Two controlled clinical studies using albendazole as the comparative agent were carried out in international sites where albendazole is approved for the treatment of strongyloidiasis of the gastrointestinal tract, and three controlled studies were carried out in the U.S. and internationally using thiabendazole as the comparative agent. Efficacy, as measured by cure rate, was defined as the absence of larvae in at least two follow-up stool examinations 3 to 4 weeks post-therapy. Based on this criterion, efficacy was significantly greater for ivermectin tablets (a single dose of 170 to 200 mcg/kg) than for albendazole (200 mg b.i.d. for 3 days). Ivermectin tablets administered as a single dose of 200 mcg/kg for 1 day was as efficacious as thiabendazole administered at 25 mg/kg b.i.d. for 3 days.
Summary of Cure Rates for Ivermectin Versus Comparative Agents in the Treatment of Strongyloidiasis
| Cure Rate*(%) | ||
| Ivermectin** | Comparative Agent | |
| Albendazole***Comparative | ||
| International Study | 24/26 (92) | 12/22 (55) |
| WHO Study | 126/152 (83) | 67/149 (45) |
| Thiabendazole†Comparative | ||
| International Study | 9/14 (64) | 13/15 (87) |
| US Studies | 14/14 (100) | 16/17 (94) |
* Number and % of evaluable patients
** 170-200 mcg/kg
*** 200 mg b.i.d. for 3 days
†25 mg/kg b.i.d. for 3 days
In one study conducted in France, a non-endemic area where there was no possibility of reinfection, several patients were observed to have recrudescence of
The evaluation of ivermectin tablets in the treatment of onchocerciasis is based on the results of clinical studies involving 1278 patients. In a double-blind, placebo-controlled study involving adult patients with moderate to severe onchocercal infection, patients who received a single dose of 150 mcg/kg ivermectin tablets experienced an 83.2% and 99.5% decrease in skin microfilariae count (geometric mean) 3 days and 3 months after the dose, respectively. A marked reduction of >90% was maintained for up to 12 months after the single dose. As with other microfilaricidal drugs, there was an increase in the microfilariae count in the anterior chamber of the eye at day 3 after treatment in some patients. However, at 3 and 6 months after the dose, a significantly greater percentage of patients treated with ivermectin tablets had decreases in microfilariae count in the anterior chamber than patients treated with placebo.
In a separate open study involving pediatric patients ages 6 to 13 (n=103; weight range: 17-41 kg), similar decreases in skin microfilariae counts were observed for up to 12 months after dosing.
Following oral administration of ivermectin, plasma concentrations are approximately proportional to the dose. In two studies, after single 12-mg doses of ivermectin tablets in fasting healthy volunteers (representing a mean dose of 165 mcg/kg), the mean peak plasma concentrations of the major component (H2B1a) were 46.6 (± 21.9) (range: 16.4-101.1) and 30.6 (± 15.6) (range: 13.9-68.4) ng/mL, respectively, at approximately 4 hours after dosing. Ivermectin is metabolized in the liver, and ivermectin and/or its metabolites are excreted almost exclusively in the feces over an estimated 12 days, with less than 1% of the administered dose excreted in the urine. The plasma half-life of ivermectin in man is approximately 18 hours following oral administration.
The safety and pharmacokinetic properties of ivermectin were further assessed in a multiple-dose clinical pharmacokinetic study involving healthy volunteers. Subjects received oral doses of 30 to 120 mg (333 to 2000 mcg/kg) ivermectin in a fasted state or 30 mg (333 to 600 mcg/kg) ivermectin following a standard high-fat (48.6 g of fat) meal. Administration of 30 mg ivermectin following a high-fat meal resulted in an approximate 2.5-fold increase in bioavailability relative to administration of 30 mg ivermectin in the fasted state.
Ivermectin is a member of the avermectin class of broad-spectrum antiparasitic agents which have a unique mode of action. Compounds of the class bind selectively and with high affinity to glutamate- gated chloride ion channels which occur in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
The selective activity of compounds of this class is attributable to the facts that some mammals do not have glutamate-gated chloride channels and that the avermectins have a low affinity for mammalian ligand-gated chloride channels. In addition, ivermectin does not readily cross the blood-brain barrier in humans.
Ivermectin is active against various life-cycle stages of many but not all nematodes. It is active against the tissue microfilariae of
Two controlled clinical studies using albendazole as the comparative agent were carried out in international sites where albendazole is approved for the treatment of strongyloidiasis of the gastrointestinal tract, and three controlled studies were carried out in the U.S. and internationally using thiabendazole as the comparative agent. Efficacy, as measured by cure rate, was defined as the absence of larvae in at least two follow-up stool examinations 3 to 4 weeks post-therapy. Based on this criterion, efficacy was significantly greater for ivermectin tablets (a single dose of 170 to 200 mcg/kg) than for albendazole (200 mg b.i.d. for 3 days). Ivermectin tablets administered as a single dose of 200 mcg/kg for 1 day was as efficacious as thiabendazole administered at 25 mg/kg b.i.d. for 3 days.
Summary of Cure Rates for Ivermectin Versus Comparative Agents in the Treatment of Strongyloidiasis
| Cure Rate*(%) | ||
| Ivermectin** | Comparative Agent | |
| Albendazole***Comparative | ||
| International Study | 24/26 (92) | 12/22 (55) |
| WHO Study | 126/152 (83) | 67/149 (45) |
| Thiabendazole†Comparative | ||
| International Study | 9/14 (64) | 13/15 (87) |
| US Studies | 14/14 (100) | 16/17 (94) |
* Number and % of evaluable patients
** 170-200 mcg/kg
*** 200 mg b.i.d. for 3 days
†25 mg/kg b.i.d. for 3 days
In one study conducted in France, a non-endemic area where there was no possibility of reinfection, several patients were observed to have recrudescence of
The evaluation of ivermectin tablets in the treatment of onchocerciasis is based on the results of clinical studies involving 1278 patients. In a double-blind, placebo-controlled study involving adult patients with moderate to severe onchocercal infection, patients who received a single dose of 150 mcg/kg ivermectin tablets experienced an 83.2% and 99.5% decrease in skin microfilariae count (geometric mean) 3 days and 3 months after the dose, respectively. A marked reduction of >90% was maintained for up to 12 months after the single dose. As with other microfilaricidal drugs, there was an increase in the microfilariae count in the anterior chamber of the eye at day 3 after treatment in some patients. However, at 3 and 6 months after the dose, a significantly greater percentage of patients treated with ivermectin tablets had decreases in microfilariae count in the anterior chamber than patients treated with placebo.
In a separate open study involving pediatric patients ages 6 to 13 (n=103; weight range: 17-41 kg), similar decreases in skin microfilariae counts were observed for up to 12 months after dosing.
Dosage Guidelines for Ivermectin Tablets for Strongyloidiasis
Body Weight (kg) | Single Oral Dose Number of 3-mg Tablets | Number of 6-mg Tablets |
15-24 | 1 tablet | ½ tablet |
25-35 | 2 tablets | 1 tablet |
36-50 | 3 tablets | 1½ tablets |
51-65 | 4 tablets | 2 tablets |
66-79 | 5 tablets | 2½ tablets |
≥ 80 | 200 mcg/kg | 200 mcg/kg |
The recommended dosage of ivermectin tablets for the treatment of onchocerciasis is a single oral dose designed to provide approximately 150 mcg of ivermectin per kg of body weight. See Table 2 for dosage guidelines. Patients should take tablets on an empty stomach with water. (See
Following oral administration of ivermectin, plasma concentrations are approximately proportional to the dose. In two studies, after single 12-mg doses of ivermectin tablets in fasting healthy volunteers (representing a mean dose of 165 mcg/kg), the mean peak plasma concentrations of the major component (H2B1a) were 46.6 (± 21.9) (range: 16.4-101.1) and 30.6 (± 15.6) (range: 13.9-68.4) ng/mL, respectively, at approximately 4 hours after dosing. Ivermectin is metabolized in the liver, and ivermectin and/or its metabolites are excreted almost exclusively in the feces over an estimated 12 days, with less than 1% of the administered dose excreted in the urine. The plasma half-life of ivermectin in man is approximately 18 hours following oral administration.
The safety and pharmacokinetic properties of ivermectin were further assessed in a multiple-dose clinical pharmacokinetic study involving healthy volunteers. Subjects received oral doses of 30 to 120 mg (333 to 2000 mcg/kg) ivermectin in a fasted state or 30 mg (333 to 600 mcg/kg) ivermectin following a standard high-fat (48.6 g of fat) meal. Administration of 30 mg ivermectin following a high-fat meal resulted in an approximate 2.5-fold increase in bioavailability relative to administration of 30 mg ivermectin in the fasted state.
Ivermectin is a member of the avermectin class of broad-spectrum antiparasitic agents which have a unique mode of action. Compounds of the class bind selectively and with high affinity to glutamate- gated chloride ion channels which occur in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
The selective activity of compounds of this class is attributable to the facts that some mammals do not have glutamate-gated chloride channels and that the avermectins have a low affinity for mammalian ligand-gated chloride channels. In addition, ivermectin does not readily cross the blood-brain barrier in humans.
Ivermectin is active against various life-cycle stages of many but not all nematodes. It is active against the tissue microfilariae of
Two controlled clinical studies using albendazole as the comparative agent were carried out in international sites where albendazole is approved for the treatment of strongyloidiasis of the gastrointestinal tract, and three controlled studies were carried out in the U.S. and internationally using thiabendazole as the comparative agent. Efficacy, as measured by cure rate, was defined as the absence of larvae in at least two follow-up stool examinations 3 to 4 weeks post-therapy. Based on this criterion, efficacy was significantly greater for ivermectin tablets (a single dose of 170 to 200 mcg/kg) than for albendazole (200 mg b.i.d. for 3 days). Ivermectin tablets administered as a single dose of 200 mcg/kg for 1 day was as efficacious as thiabendazole administered at 25 mg/kg b.i.d. for 3 days.
Summary of Cure Rates for Ivermectin Versus Comparative Agents in the Treatment of Strongyloidiasis
| Cure Rate*(%) | ||
| Ivermectin** | Comparative Agent | |
| Albendazole***Comparative | ||
| International Study | 24/26 (92) | 12/22 (55) |
| WHO Study | 126/152 (83) | 67/149 (45) |
| Thiabendazole†Comparative | ||
| International Study | 9/14 (64) | 13/15 (87) |
| US Studies | 14/14 (100) | 16/17 (94) |
* Number and % of evaluable patients
** 170-200 mcg/kg
*** 200 mg b.i.d. for 3 days
†25 mg/kg b.i.d. for 3 days
In one study conducted in France, a non-endemic area where there was no possibility of reinfection, several patients were observed to have recrudescence of
The evaluation of ivermectin tablets in the treatment of onchocerciasis is based on the results of clinical studies involving 1278 patients. In a double-blind, placebo-controlled study involving adult patients with moderate to severe onchocercal infection, patients who received a single dose of 150 mcg/kg ivermectin tablets experienced an 83.2% and 99.5% decrease in skin microfilariae count (geometric mean) 3 days and 3 months after the dose, respectively. A marked reduction of >90% was maintained for up to 12 months after the single dose. As with other microfilaricidal drugs, there was an increase in the microfilariae count in the anterior chamber of the eye at day 3 after treatment in some patients. However, at 3 and 6 months after the dose, a significantly greater percentage of patients treated with ivermectin tablets had decreases in microfilariae count in the anterior chamber than patients treated with placebo.
In a separate open study involving pediatric patients ages 6 to 13 (n=103; weight range: 17-41 kg), similar decreases in skin microfilariae counts were observed for up to 12 months after dosing.
Dosage Guidelines for Ivermectin Tablets for Onchocerciasis
Body Weight (kg) | Single Oral Dose Number of 3-mg Tablets | Number of 6-mg Tablets |
15-24 | 1 tablet | ½ tablet |
26-44 | 2 tablets | 1 tablet |
45-64 | 3 tablets | 1½ tablets |
65-84 | 4 tablets | 2 tablets |
≥ 85 | 150 mcg/kg | 150 mcg/kg |
Ivermectin tablets are contraindicated in patients who are hypersensitive to any component of this product.
In four clinical studies involving a total of 109 patients given either one or two doses of 170 to 200 mcg/kg of ivermectin tablets, the following adverse reactions were reported as possibly, probably, or definitely related to ivermectin tablets:
In comparative trials, patients treated with ivermectin tablets experienced more abdominal distention and chest discomfort than patients treated with albendazole. However, ivermectin tablet was better tolerated than thiabendazole in comparative studies involving 37 patients treated with thiabendazole.
The Mazzotti-type and ophthalmologic reactions associated with the treatment of onchocerciasis or the disease itself would not be expected to occur in strongyloidiasis patients treated with ivermectin tablets. (See
In four clinical studies involving a total of 109 patients given either one or two doses of 170 to 200 mcg/kg of ivermectin tablets, the following adverse reactions were reported as possibly, probably, or definitely related to ivermectin tablets:
In comparative trials, patients treated with ivermectin tablets experienced more abdominal distention and chest discomfort than patients treated with albendazole. However, ivermectin tablet was better tolerated than thiabendazole in comparative studies involving 37 patients treated with thiabendazole.
The Mazzotti-type and ophthalmologic reactions associated with the treatment of onchocerciasis or the disease itself would not be expected to occur in strongyloidiasis patients treated with ivermectin tablets. (See ADVERSE REACTIONS,
In clinical trials involving 109 patients given either one or two doses of 170 to 200 mcg/kg ivermectin tablets, the following laboratory abnormalities were seen regardless of drug relationship: elevation in ALT and/or AST (2%), decrease in leukocyte count (3%). Leukopenia and anemia were seen in one patient.
In clinical trials involving 963 adult patients treated with 100 to 200 mcg/kg ivermectin tablets, worsening of the following Mazzotti reactions during the first 4 days post-treatment were reported: arthralgia/synovitis (9.3%), axillary lymph node enlargement and tenderness (11.0% and 4.4%, respectively), cervical lymph node enlargement and tenderness (5.3% and 1.2%, respectively), inguinal lymph node enlargement and tenderness (12.6% and 13.9%, respectively), other lymph node enlargement and tenderness (3.0% and 1.9%, respectively), pruritus (27.5%), skin involvement including edema, papular and pustular or frank urticarial rash (22.7%), and fever (22.6%). (See WARNINGS.)
In clinical trials, ophthalmological conditions were examined in 963 adult patients before treatment, at day 3, and months 3 and 6 after treatment with 100 to 200 mcg/kg ivermectin tablets. Changes observed were primarily deterioration from baseline 3 days post-treatment. Most changes either returned to baseline condition or improved over baseline severity at the month 3 and 6 visits. The percentages of patients with worsening of the following conditions at day 3, month 3 and 6, respectively, were: limbitis: 5.5%, 4.8%, and 3.5% and punctate opacity: 1.8%, 1.8%, and 1.4%. The corresponding percentages for patients treated with placebo were: limbitis: 6.2%, 9.9%, and 9.4% and punctate opacity: 2.0%, 6.4%, and 7.2%. (See WARNINGS.)
In clinical trials involving 963 adult patients who received 100 to 200 mcg/kg ivermectin tablets, the following clinical adverse reactions were reported as possibly, probably, or definitely related to the drug in ≥1% of the patients: facial edema (1.2%), peripheral edema (3.2%), orthostatic hypotension (1.1%), and tachycardia (3.5%). Drug-related headache and myalgia occurred in <1% of patients (0.2% and 0.4%, respectively). However, these were the most common adverse experiences reported overall during these trials regardless of causality (22.3% and 19.7%, respectively).
A similar safety profile was observed in an open study in pediatric patients ages 6 to 13.
The following ophthalmological side effects do occur due to the disease itself but have also been reported after treatment with ivermectin tablets: abnormal sensation in the eyes, eyelid edema, anterior uveitis, conjunctivitis, limbitis, keratitis, and chorioretinitis or choroiditis. These have rarely been severe or associated with loss of vision and have generally resolved without corticosteroid treatment.
In controlled clinical trials, the following laboratory adverse experiences were reported as possibly, probably, or definitely related to the drug in ≥1% of the patients: eosinophilia (3%) and hemoglobin increase (1%).
The following adverse reactions have been reported since the drug was registered overseas:
Conjunctival hemorrhage
Hypotension (mainly orthostatic hypotension), worsening of bronchial asthma, toxic epidermal necrolysis, Stevens-Johnson syndrome, seizures, hepatitis, elevation of liver enzymes, and elevation of bilirubin.
Neurotoxicity including alteration of consciousness of variable severity (e.g., somnolence/drowsiness, stupor, and coma), confusion, disorientation, and death (see WARNINGS).
In clinical trials involving 109 patients given either one or two doses of 170 to 200 mcg/kg ivermectin tablets, the following laboratory abnormalities were seen regardless of drug relationship: elevation in ALT and/or AST (2%), decrease in leukocyte count (3%). Leukopenia and anemia were seen in one patient.
In clinical trials involving 963 adult patients treated with 100 to 200 mcg/kg ivermectin tablets, worsening of the following Mazzotti reactions during the first 4 days post-treatment were reported: arthralgia/synovitis (9.3%), axillary lymph node enlargement and tenderness (11.0% and 4.4%, respectively), cervical lymph node enlargement and tenderness (5.3% and 1.2%, respectively), inguinal lymph node enlargement and tenderness (12.6% and 13.9%, respectively), other lymph node enlargement and tenderness (3.0% and 1.9%, respectively), pruritus (27.5%), skin involvement including edema, papular and pustular or frank urticarial rash (22.7%), and fever (22.6%). (See
Historical data have shown that microfilaricidal drugs, such as diethylcarbamazine citrate (DEC-C), might cause cutaneous and/or systemic reactions of varying severity (the Mazzotti reaction) and ophthalmological reactions in patients with onchocerciasis. These reactions are probably due to allergic and inflammatory responses to the death of microfilariae. Patients treated with ivermectin tablets for onchocerciasis may experience these reactions in addition to clinical adverse reactions possibly, probably, or definitely related to the drug itself. (See ADVERSE REACTIONS,
The treatment of severe Mazzotti reactions has not been subjected to controlled clinical trials. Oral hydration, recumbency, intravenous normal saline, and/ or parenteral corticosteroids have been used to treat postural hypotension. Antihistamines and/or aspirin have been used for most mild to moderate cases.
Neurotoxicity with the use of ivermectin, including alteration of consciousness of variable severity (e.g., somnolence/drowsiness, stupor, and coma), confusion, disorientation and death, has been reported in patients without onchocerciasis or in patients with onchocerciasis in the absence of
In clinical trials, ophthalmological conditions were examined in 963 adult patients before treatment, at day 3, and months 3 and 6 after treatment with 100 to 200 mcg/kg ivermectin tablets. Changes observed were primarily deterioration from baseline 3 days post-treatment. Most changes either returned to baseline condition or improved over baseline severity at the month 3 and 6 visits. The percentages of patients with worsening of the following conditions at day 3, month 3 and 6, respectively, were: limbitis: 5.5%, 4.8%, and 3.5% and punctate opacity: 1.8%, 1.8%, and 1.4%. The corresponding percentages for patients treated with placebo were: limbitis: 6.2%, 9.9%, and 9.4% and punctate opacity: 2.0%, 6.4%, and 7.2%. (See
Historical data have shown that microfilaricidal drugs, such as diethylcarbamazine citrate (DEC-C), might cause cutaneous and/or systemic reactions of varying severity (the Mazzotti reaction) and ophthalmological reactions in patients with onchocerciasis. These reactions are probably due to allergic and inflammatory responses to the death of microfilariae. Patients treated with ivermectin tablets for onchocerciasis may experience these reactions in addition to clinical adverse reactions possibly, probably, or definitely related to the drug itself. (See ADVERSE REACTIONS,
The treatment of severe Mazzotti reactions has not been subjected to controlled clinical trials. Oral hydration, recumbency, intravenous normal saline, and/ or parenteral corticosteroids have been used to treat postural hypotension. Antihistamines and/or aspirin have been used for most mild to moderate cases.
Neurotoxicity with the use of ivermectin, including alteration of consciousness of variable severity (e.g., somnolence/drowsiness, stupor, and coma), confusion, disorientation and death, has been reported in patients without onchocerciasis or in patients with onchocerciasis in the absence of
In clinical trials involving 963 adult patients who received 100 to 200 mcg/kg ivermectin tablets, the following clinical adverse reactions were reported as possibly, probably, or definitely related to the drug in ≥1% of the patients: facial edema (1.2%), peripheral edema (3.2%), orthostatic hypotension (1.1%), and tachycardia (3.5%). Drug-related headache and myalgia occurred in <1% of patients (0.2% and 0.4%, respectively). However, these were the most common adverse experiences reported overall during these trials regardless of causality (22.3% and 19.7%, respectively).
A similar safety profile was observed in an open study in pediatric patients ages 6 to 13.
The following ophthalmological side effects do occur due to the disease itself but have also been reported after treatment with ivermectin tablets: abnormal sensation in the eyes, eyelid edema, anterior uveitis, conjunctivitis, limbitis, keratitis, and chorioretinitis or choroiditis. These have rarely been severe or associated with loss of vision and have generally resolved without corticosteroid treatment.
In controlled clinical trials, the following laboratory adverse experiences were reported as possibly, probably, or definitely related to the drug in ≥1% of the patients: eosinophilia (3%) and hemoglobin increase (1%).
The following adverse reactions have been reported since the drug was registered overseas:
Conjunctival hemorrhage
Hypotension (mainly orthostatic hypotension), worsening of bronchial asthma, toxic epidermal necrolysis, Stevens-Johnson syndrome, seizures, hepatitis, elevation of liver enzymes, and elevation of bilirubin.
Neurotoxicity including alteration of consciousness of variable severity (e.g., somnolence/drowsiness, stupor, and coma), confusion, disorientation, and death (see
Historical data have shown that microfilaricidal drugs, such as diethylcarbamazine citrate (DEC-C), might cause cutaneous and/or systemic reactions of varying severity (the Mazzotti reaction) and ophthalmological reactions in patients with onchocerciasis. These reactions are probably due to allergic and inflammatory responses to the death of microfilariae. Patients treated with ivermectin tablets for onchocerciasis may experience these reactions in addition to clinical adverse reactions possibly, probably, or definitely related to the drug itself. (See ADVERSE REACTIONS,
The treatment of severe Mazzotti reactions has not been subjected to controlled clinical trials. Oral hydration, recumbency, intravenous normal saline, and/ or parenteral corticosteroids have been used to treat postural hypotension. Antihistamines and/or aspirin have been used for most mild to moderate cases.
Neurotoxicity with the use of ivermectin, including alteration of consciousness of variable severity (e.g., somnolence/drowsiness, stupor, and coma), confusion, disorientation and death, has been reported in patients without onchocerciasis or in patients with onchocerciasis in the absence of
Ivermectin tablet is a semisynthetic, anthelmintic agent for oral administration. Ivermectin is derived from the avermectins, a class of highly active broad-spectrum, anti-parasitic agents isolated from the fermentation products of
1aand less than 10% 5-
1a, generally referred to as 22,23- dihydroavermectin B
1aand B
1b, or H
2B
1aand H
2B
1b, respectively. The respective empirical formulas are C
48H
74O
14and C
47H
72O
14, with molecular weights of 875.10 and 861.07, respectively. The structural formulas are:
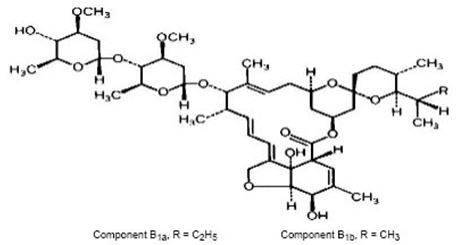
Ivermectin is a white to yellowish-white, nonhygroscopic, crystalline powder with a melting point of about 155°C. It is insoluble in water but is freely soluble in methanol and soluble in 95% ethanol.
Ivermectin tablets are available in 3 mg and 6 mg tablets containing the following inactive ingredients: microcrystalline cellulose, croscarmellose sodium, colloidal silicon dioxide, pregelatinized starch, magnesium stearate.
Following oral administration of ivermectin, plasma concentrations are approximately proportional to the dose. In two studies, after single 12-mg doses of ivermectin tablets in fasting healthy volunteers (representing a mean dose of 165 mcg/kg), the mean peak plasma concentrations of the major component (H
2B
1a) were 46.6 (± 21.9) (range: 16.4-101.1) and 30.6 (± 15.6) (range: 13.9-68.4) ng/mL, respectively, at approximately 4 hours after dosing. Ivermectin is metabolized in the liver, and ivermectin and/or its metabolites are excreted almost exclusively in the feces over an estimated 12 days, with less than 1% of the administered dose excreted in the urine. The plasma half-life of ivermectin in man is approximately 18 hours following oral administration.
The safety and pharmacokinetic properties of ivermectin were further assessed in a multiple-dose clinical pharmacokinetic study involving healthy volunteers. Subjects received oral doses of 30 to 120 mg (333 to 2000 mcg/kg) ivermectin in a fasted state or 30 mg (333 to 600 mcg/kg) ivermectin following a standard high-fat (48.6 g of fat) meal. Administration of 30 mg ivermectin following a high-fat meal resulted in an approximate 2.5-fold increase in bioavailability relative to administration of 30 mg ivermectin in the fasted state.
Ivermectin is a member of the avermectin class of broad-spectrum antiparasitic agents which have a unique mode of action. Compounds of the class bind selectively and with high affinity to glutamate- gated chloride ion channels which occur in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
The selective activity of compounds of this class is attributable to the facts that some mammals do not have glutamate-gated chloride channels and that the avermectins have a low affinity for mammalian ligand-gated chloride channels. In addition, ivermectin does not readily cross the blood-brain barrier in humans.
Ivermectin is active against various life-cycle stages of many but not all nematodes. It is active against the tissue microfilariae of
Two controlled clinical studies using albendazole as the comparative agent were carried out in international sites where albendazole is approved for the treatment of strongyloidiasis of the gastrointestinal tract, and three controlled studies were carried out in the U.S. and internationally using thiabendazole as the comparative agent. Efficacy, as measured by cure rate, was defined as the absence of larvae in at least two follow-up stool examinations 3 to 4 weeks post-therapy. Based on this criterion, efficacy was significantly greater for ivermectin tablets (a single dose of 170 to 200 mcg/kg) than for albendazole (200 mg b.i.d. for 3 days). Ivermectin tablets administered as a single dose of 200 mcg/kg for 1 day was as efficacious as thiabendazole administered at 25 mg/kg b.i.d. for 3 days.
Summary of Cure Rates for Ivermectin Versus Comparative Agents in the Treatment of Strongyloidiasis
| | Cure Rate*(%) | |
| Ivermectin ** | Comparative Agent | |
| Albendazole ***Comparative | | |
| International Study | 24/26 (92) | 12/22 (55) |
| WHO Study | 126/152 (83) | 67/149 (45) |
| Thiabendazole †Comparative | | |
| International Study | 9/14 (64) | 13/15 (87) |
| US Studies | 14/14 (100) | 16/17 (94) |
* Number and % of evaluable patients
** 170-200 mcg/kg
*** 200 mg b.i.d. for 3 days
†25 mg/kg b.i.d. for 3 days
In one study conducted in France, a non-endemic area where there was no possibility of reinfection, several patients were observed to have recrudescence of
The evaluation of ivermectin tablets in the treatment of onchocerciasis is based on the results of clinical studies involving 1278 patients. In a double-blind, placebo-controlled study involving adult patients with moderate to severe onchocercal infection, patients who received a single dose of 150 mcg/kg ivermectin tablets experienced an 83.2% and 99.5% decrease in skin microfilariae count (geometric mean) 3 days and 3 months after the dose, respectively. A marked reduction of >90% was maintained for up to 12 months after the single dose. As with other microfilaricidal drugs, there was an increase in the microfilariae count in the anterior chamber of the eye at day 3 after treatment in some patients. However, at 3 and 6 months after the dose, a significantly greater percentage of patients treated with ivermectin tablets had decreases in microfilariae count in the anterior chamber than patients treated with placebo.
In a separate open study involving pediatric patients ages 6 to 13 (n=103; weight range: 17-41 kg), similar decreases in skin microfilariae counts were observed for up to 12 months after dosing.