Ketoconazole
Ketoconazole Prescribing Information
Ketoconazole Cream, 2% is indicated for the topical treatment of tinea corporis, tinea cruris and tinea pedis caused by
If a patient shows no clinical improvement after the treatment period, the diagnosis should be redetermined.
Ketoconazole Cream, 2% is contraindicated in persons who have shown hypersensitivity to the active or excipient ingredients of this formulation.
During clinical trials 45 (5%) of 905 patients treated with ketoconazole cream, 2% and 5 (2.4%) of 208 patients treated with placebo reported side effects consisting mainly of severe irritation, pruritus and stinging. One of the patients treated with ketoconazole cream developed a painful allergic reaction.
In worldwide postmarketing experience, rare reports of contact dermatitis have been associated with ketoconazole cream or one of its excipients, namely sodium sulfite or propylene glycol.
Ketoconazole Cream, 2% contains the broad-spectrum synthetic antifungal agent, ketoconazole 2%. Each gram, for topical administration, contains ketoconazole 20 mg and is formulated in an aqueous cream vehicle consisting of propylene glycol, purified water, cetyl alcohol, stearyl alcohol, isopropyl myristate, sorbitan monostearate, polysorbate 60, polysorbate 80, and sodium sulfite, anhydrous:
Ketoconazole is
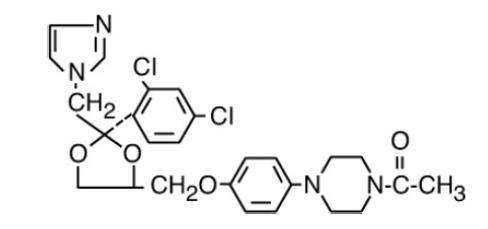
C26H28Cl2N4O4 M.W. 531.44
When ketoconazole cream, 2% was applied dermally to intact or abraded skin of Beagle dogs for 28 consecutive days at a dose of 80 mg, there were no detectable plasma levels using an assay method having a lower detection limit of 2 ng/ml.
After a single topical application to the chest, back and arms of normal volunteers, systemic absorption of ketoconazole was not detected at the 5 ng/ml level in blood over a 72-hour period.
Two dermal irritancy studies, a human sensitization test, a phototoxicity study and a photoallergy study conducted in 38 male and 62 female volunteers showed no contact sensitization of the delayed hypersensitivity type, no irritation, no phototoxicity and no photoallergenic potential due to ketoconazole cream, 2%.