Ketoconazole
Ketoconazole Prescribing Information
Ketoconazole shampoo, 2% is indicated for the treatment of tinea (pityriasis) versicolor caused by or presumed to be caused by
Apply the shampoo to the damp skin of the affected area and a wide margin surrounding this area. Lather, leave in place for 5 minutes and then rinse off with water.
One application of the shampoo should be sufficient.
Ketoconazole shampoo, 2% is contraindicated in persons who have known hypersensitivity to the active ingredient or excipients of this formulation.
In 11 double-blind trials in 264 patients using ketoconazole shampoo, 2% for the treatment of dandruff or seborrheic dermatitis, an increase in normal hair loss and irritation occurred in less than 1% of patients. In three open-label safety trials in which 41 patients shampooed 4 times to 10 times weekly for six months, the following adverse experiences each occurred once: abnormal hair texture, scalp pustules, mild dryness of the skin and itching. As with other shampoos, oiliness and dryness of hair and scalp have been reported. In a double-blind, placebo-controlled trial in which patients with tinea versicolor were treated with either a single application of ketoconazole shampoo, 2% (n=106), a daily application for three consecutive days (n=107) or placebo (n=105), drug-related adverse events occurred in 5 (5%), 7 (7%) and 4 (4%) of patients, respectively. The only events that occurred in more than one patient in any one of the three treatment groups were pruritus, application site reaction and dry skin; none of these events occurred in more than 3% of the patients in any one of the three groups.
Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency. The following adverse drug reactions have been identified during post-marketing experience with ketoconazole shampoo, 2%: there have been reports of hair discoloration and abnormal hair texture, itching, skin burning sensation, contact dermatitis, hypersensitivity, angioedema, alopecia, rash, urticaria, skin irritation, dry skin and application site reactions.
Ketoconazole shampoo, 2% is a red-orange liquid for topical application, containing the broad spectrum synthetic antifungal agent ketoconazole in a concentration of 2% in an aqueous suspension. It also contains: cocodiethanolamide, disodium laureth sulfosuccinate, FD&C red no. 40, hydrochloric acid, imidurea, laurdimonium hydroxypropyl hydrolyzed animal collagen, PEG-120 methyl glucose dioleate, purified water, sodium chloride, sodium hydroxide and sodium lauryl ether sulfate.
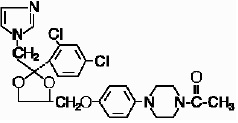
Ketoconazole is
Tinea (pityriasis) versicolor is a non-contagious infection of the skin caused by
Ketoconazole was not detected in plasma in 39 patients who shampooed 4 times to 10 times per week for 6 months or in 33 patients who shampooed 2 times to 3 times per week for 3 months to 26 months (mean: 16 months).
An exaggerated use washing test on the sensitive antecubital skin of 10 subjects twice daily for five consecutive days showed that the irritancy potential of ketoconazole shampoo, 2% was significantly less than that of 2.5% selenium sulfide shampoo.
A human sensitization test, a phototoxicity study and a photoallergy study conducted in 38 male and 22 female volunteers showed no contact sensitization of the delayed hypersensitivity type, no phototoxicity and no photoallergenic potential due to ketoconazole shampoo, 2%.
Interpretations of
Ketoconazole is a broad spectrum synthetic antifungal agent which inhibits the growth of the following common dermatophytes and yeasts by altering the permeability of the cell membrane: dermatophytes: