Kit For The Preparation Of Technetium Tc 99m Medronate
(Tc 99m Medronate)Kit For The Preparation Of Technetium Tc 99m Medronate Prescribing Information
Technetium Tc 99m Medronate Injection may be used as a bone imaging agent to delineate areas of altered osteogenesis.
Shielding should be utilized when preparing Technetium Tc 99m Medronate Injection.
After preparation with oxidant-free Sodium Pertechnetate Tc 99m Injection, the suggested dose range of Technetium Tc 99m Medronate Injection in the average ADULT patient (70 kg.) is:
- 370-740 megabecquerels: (10-20 millicuries) given intravenously.
- Imaging is optimal at 1 to 4 hours post Injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
None known.
Several adverse reactions due to Technetium Tc 99m Medronate Injection have been reported. These were usually hypersensitivity reactions characterized by itching, various skin rashes, hypotension, chills, nausea, and vomiting. There have also been rare cases of dizziness and asthenia associated with the use of Technetium Tc 99m Medronate.
Kit for the Preparation of Technetium Tc 99m Medronate is a multidose reaction vial which contains the sterile, non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Medronate Injection for diagnostic use by Intravenous injection.
Each 10mL multidose vial contains:
- Medronic acid: 20 mg
- Ascorbic acid: 1 mg
- Stannous fluoride, SnF
2: 0.13 mg (minimum) - Total tin (maximum, as stannous fluoride, SnF
2): 0.38 mg
The pH is adjusted to 6.5 (6.3 to 6.7) with sodium hydroxide and/or hydrochloric acid prior to lyophilization. No bacteriostatic preservative is present in the vial. The contents of the vial are lyophilized and sealed under nitrogen at the time of manufacture. The structural formula is:
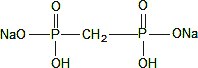
When a solution of sterile, non-pyrogenic, oxidant-free Sodium Pertechnetate Tc 99m Injection is added to the vial, the diagnostic agent, Technetium Tc 99m Medronate is formed for administration by intravenous injection. The pH of the reconstituted product is 5.4 to 6.8. The precise structure of Technetium Tc 99m Medronate Injection is not known at this time.
During the initial 24 hours following intravenous injection of Technetium Tc 99m Medronate, about 50% of each dose is retained in the skeleton, and about 50% is excreted in the urine. Upon intravenous injection, Technetium Tc 99m Medronate exhibits a specific affinity for areas of altered osteogenesis. In humans, blood levels fall to 4 to 10% of the injected dose by two hours post-injection and to 3 to 5% by three hours.
Uptake of Technetium Tc 99m Medronate Injection in bone appears to be related to osteogenic activity and to skeletal blood perfusion. The deposition in the skeleton is bilaterally symmetrical, with increased accumulation in the axial structure as compared to the appendicular skeleton. There is increased activity in the distal aspect on long bones as compared to the diaphyses.