Labetalol Hydrochloride
Labetalol Hydrochloride Prescribing Information
Labetalol hydrochloride tablets, USP are indicated in the management of hypertension. Labetalol hydrochloride tablets, USP may be used alone or in combination with other antihypertensive agents, especially thiazide and loop diuretics.
DOSAGE MUST BE INDIVIDUALIZED. The recommended
Since the full antihypertensive effect of labetalol hydrochloride tablets is usually seen within the first 1 to 3 hours of the initial dose or dose increment, the assurance of a lack of an exaggerated hypotensive response can be clinically established in the office setting. The antihypertensive effects of continued dosing can be measured at subsequent visits, approximately 12 hours after a dose, to determine whether further titration is necessary.
Patients with severe hypertension may require from 1200 mg to 2400 mg per day, with or without thiazide diuretics. Should side effects (principally nausea or dizziness) occur with these doses administered b.i.d. (twice daily), the same total daily dose administered t.i.d. (three times daily) may improve tolerability and facilitate further titration. Titration increments should not exceed 200 mg b.i.d. (twice daily).
When a diuretic is added, an additive antihypertensive effect can be expected. In some cases this may necessitate a labetalol hydrochloride tablet dosage adjustment. As with most antihypertensive drugs, optimal dosages of labetalol hydrochloride tablets are usually lower in patients also receiving a diuretic.
When transferring patients from other antihypertensive drugs, labetalol hydrochloride tablets should be introduced as recommended and the dosage of the existing therapy progressively decreased.
As in the general patient population, labetalol therapy may be initiated at 100 mg twice daily and titrated upwards in increments of 100 mg b.i.d. (twice daily) as required for control of blood pressure. Since some elderly patients eliminate labetalol more slowly, however, adequate control of blood pressure may be achieved at a lower maintenance dosage compared to the general population. The majority of elderly patients will require between 100 mg and 200 mg b.i.d. (twice daily).
Labetalol hydrochloride is contraindicated in bronchial asthma, overt cardiac failure, greater-than-first-degree heart block, cardiogenic shock, severe bradycardia, other conditions associated with severe and prolonged hypotension, and in patients with a history of hypersensitivity to any component of the product
Severe hepatocellular injury, confirmed by rechallenge in at least one case, occurs rarely with labetalol therapy. The hepatic injury is usually reversible, but hepatic necrosis and death have been reported. Injury has occurred after both short- and long-term treatment and may be slowly progressive despite minimal symptomatology. Similar hepatic events have been reported with a related research compound, dilevalol HCl, including two deaths. Dilevalol HCl is one of the four isomers of labetalol hydrochloride. Thus, for patients taking labetalol, periodic determination of suitable hepatic laboratory tests would be appropriate. Appropriate laboratory testing should be done at the first symptom or sign of liver dysfunction (e.g., pruritus, dark urine, persistent anorexia, jaundice, right upper quadrant tenderness, or unexplained “flu-like” symptoms). If the patient has laboratory evidence of liver injury or jaundice, labetalol should be stopped and not restarted.
Sympathetic stimulation is a vital component supporting circulatory function in congestive heart failure. Beta-blockade carries a potential hazard of further depressing myocardial contractility and precipitating more severe failure. Although beta-blockers should be avoided in overt congestive heart failure, if necessary, labetalol hydrochloride can be used with caution in patients with a history of heart failure who are well compensated. Congestive heart failure has been observed in patients receiving labetalol hydrochloride. Labetalol hydrochloride does not abolish the inotropic action of digitalis on heart muscle.
In patients with latent cardiac insufficiency, continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. At the first sign or symptom of impending cardiac failure, patients should be fully digitalized and/or be given a diuretic, and the response should be observed closely. If cardiac failure continues, despite adequate digitalization and diuretic, therapy with labetalol hydrochloride should be withdrawn (gradually, if possible).
Angina pectoris has not been reported upon labetalol hydrochloride discontinuation. However, hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy; exacerbation of angina and, in some cases, myocardial infarction have occurred after
Labetalol hydrochloride has been shown to be effective in lowering blood pressure and relieving symptoms in patients with pheochromocytoma. However, paradoxical hypertensive responses have been reported in a few patients with this tumor; therefore, use caution when administering labetalol hydrochloride to patients with pheochromocytoma.
Beta-adrenergic blockade may prevent the appearance of premonitory signs and symptoms (e.g., tachycardia) of acute hypoglycemia. This is especially important with labile diabetics. Beta-blockade also reduces the release of insulin in response to hyperglycemia; it may therefore be necessary to adjust the dose of antidiabetic drugs.
Do not routinely withdraw chronic beta-blocker therapy prior to surgery. The effect of labetalol hydrochloride’s alpha-adrenergic activity has not been evaluated in this setting.
A synergism between labetalol hydrochloride and halothane anesthesia has been shown
Beta-blockers, even those with apparent cardioselectivity, should not be used in patients with a history of obstructive airway disease, including asthma.
Most adverse effects are mild and transient and occur early in the course of treatment. In controlled clinical trials of 3 to 4 months’ duration, discontinuation of labetalol hydrochloride due to one or more adverse effects was required in 7% of all patients. In these same trials, other agents with solely beta-blocking activity used in the control groups led to discontinuation in 8% to 10% of patients, and a centrally acting alpha-agonist led to discontinuation in 30% of patients.
The incidence rates of adverse reactions listed in the following table were derived from multicenter, controlled clinical trials comparing labetalol hydrochloride, placebo, metoprolol, and propranolol over treatment periods of 3 and 4 months. Where the frequency of adverse effects for labetalol hydrochloride and placebo is similar, causal relationship is uncertain. The rates are based on adverse reactions considered probably drug related by the investigator. If all reports are considered, the rates are somewhat higher (e.g., dizziness, 20%; nausea, 14%; fatigue, 11%), but the overall conclusions are unchanged.
Labetalol Hydrochloride (N=227) % | Placebo (N=98) % | Propranolol (N=84) % | Metoprolol (N=49) % | |
Body as a whole | ||||
| Fatigue | 5 | 0 | 12 | 12 |
| Asthenia | 1 | 1 | 1 | 0 |
| Headache | 2 | 1 | 1 | 2 |
Gastrointestinal | ||||
| Nausea | 6 | 1 | 1 | 2 |
| Vomiting | less than 1 | 0 | 0 | 0 |
| Dyspepsia | 3 | 1 | 1 | 0 |
| Abdominal pain | 0 | 0 | 1 | 2 |
| Diarrhea | less than 1 | 0 | 2 | 0 |
| Taste distortion | 1 | 0 | 0 | 0 |
Central and Peripheral Nervous Systems | ||||
| Dizziness | 11 | 3 | 4 | 4 |
| Paresthesia | less than 1 | 0 | 0 | 0 |
| Drowsiness | less than 1 | 2 | 2 | 2 |
Autonomic Nervous System | ||||
| Nasal stuffiness | 3 | 0 | 0 | 0 |
| Ejaculation failure | 2 | 0 | 0 | 0 |
| Impotence | 1 | 0 | 1 | 3 |
| Increased sweating | less than 1 | 0 | 0 | 0 |
Cardiovascular | ||||
| Edema | 1 | 0 | 0 | 0 |
| Postural hypotension | 1 | 0 | 0 | 0 |
| Bradycardia | 0 | 0 | 5 | 12 |
Respiratory | ||||
| Dyspnea | 2 | 0 | 1 | 2 |
Skin | ||||
| Rash | 1 | 0 | 0 | 0 |
Special Senses | ||||
| Vision abnormality | 1 | 0 | 0 | 0 |
| Vertigo | 2 | 1 | 0 | 0 |
The adverse effects were reported spontaneously and are representative of the incidence of adverse effects that may be observed in a properly selected hypertensive patient population, i.e., a group excluding patients with bronchospastic disease, overt congestive heart failure, or other contraindications to beta-blocker therapy.
Clinical trials also included studies utilizing daily doses up to 2400 mg in more severely hypertensive patients. Certain of the side effects increased with increasing dose, as shown in the following table that depicts the entire U.S. therapeutic trials data base for adverse reactions that are clearly or possibly dose related.
Labetalol Hydrochloride Daily Dose (mg) | 200 | 300 | 400 | 600 | 800 | 900 | 1200 | 1600 | 2400 |
| Number of Patients | 522 | 181 | 606 | 608 | 503 | 117 | 411 | 242 | 175 |
| Dizziness (%) | 2 | 3 | 3 | 3 | 5 | 1 | 9 | 13 | 16 |
| Fatigue | 2 | 1 | 4 | 4 | 5 | 3 | 7 | 6 | 10 |
| Nausea | less than 1 | 0 | 1 | 2 | 4 | 0 | 7 | 11 | 19 |
| Vomiting | 0 | 0 | less than 1 | less than 1 | less than 1 | 0 | 1 | 2 | 3 |
| Dyspepsia | 1 | 0 | 2 | 1 | 1 | 0 | 2 | 2 | 4 |
| Paresthesias | 2 | 0 | 2 | 2 | 1 | 1 | 2 | 5 | 5 |
| Nasal Stuffiness | 1 | 1 | 2 | 2 | 2 | 2 | 4 | 5 | 6 |
| Ejaculation Failure | 0 | 2 | 1 | 2 | 3 | 0 | 4 | 3 | 5 |
| Impotence | 1 | 1 | 1 | 1 | 2 | 4 | 3 | 4 | 3 |
| Edema | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 2 |
In addition, a number of other less common adverse events have been reported:
Fever.
Hypotension, and rarely, syncope, bradycardia, heart block.
Paresthesia, most frequently described as scalp tingling.
In most cases, it was mild and transient and usually occurred at the beginning of treatment.
Systemic lupus erythematosus, positive antinuclear factor.
Dry eyes.
Antimitochondrial antibodies.
Hepatic necrosis, hepatitis, cholestatic jaundice, elevated liver function tests.
Muscle cramps, toxic myopathy.
Bronchospasm.
Rashes of various types, such as generalized maculopapular, lichenoid, urticarial, bullous lichen planus, psoriaform, and facial erythema; Peyronie’s disease, reversible alopecia.
Difficulty in micturition, including acute urinary bladder retention.
Rare reports of hypersensitivity (e.g., rash, urticaria, pruritus, angioedema, dyspnea) and anaphylactoid reactions.
Following approval for marketing in the United Kingdom, a monitored release survey involving approximately 6,800 patients was conducted for further safety and efficacy evaluation of this product. Results of this survey indicate that the type, severity, and incidence of adverse effects were comparable to those cited above.
In addition, other adverse effects not listed above have been reported with other beta-adrenergic blocking agents.
Reversible mental depression progressing to catatonia, an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on psychometrics.
Intensification of A-V block
Labetalol hydrochloride is contraindicated in bronchial asthma, overt cardiac failure, greater-than-first-degree heart block, cardiogenic shock, severe bradycardia, other conditions associated with severe and prolonged hypotension, and in patients with a history of hypersensitivity to any component of the product
Beta-blockers, even those with apparent cardioselectivity, should not be used in patients with a history of obstructive airway disease, including asthma.
Fever combined with aching and sore throat, laryngospasm, respiratory distress.
Agranulocytosis, thrombocytopenic or nonthrombocytopenic purpura.
Mesenteric artery thrombosis, ischemic colitis.
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with labetalol hydrochloride.
There have been reversible increases of serum transaminases in 4% of patients treated with labetalol hydrochloride and tested and, more rarely, reversible increases in blood urea.
In one survey, 2.3% of patients taking labetalol hydrochloride in combination with tricyclic antidepressants experienced tremor, as compared to 0.7% reported to occur with labetalol hydrochloride alone. The contribution of each of the treatments to this adverse reaction is unknown, but the possibility of a drug interaction cannot be excluded.
Drugs possessing beta-blocking properties can blunt the bronchodilator effect of beta-receptor agonist drugs in patients with bronchospasm; therefore, doses greater than the normal anti-asthmatic dose of beta-agonist bronchodilator drugs may be required.
Cimetidine has been shown to increase the bioavailability of labetalol hydrochloride. Since this could be explained either by enhanced absorption or by an alteration of hepatic metabolism of labetalol hydrochloride, special care should be used in establishing the dose required for blood pressure control in such patients.
Synergism has been shown between halothane anesthesia and intravenously administered labetalol hydrochloride. During controlled hypotensive anesthesia using labetalol hydrochloride in association with halothane, high concentrations (3% or above) of halothane should not be used because the degree of hypotension will be increased and because of the possibility of a large reduction in cardiac output and an increase in central venous pressure. The anesthesiologist should be informed when a patient is receiving labetalol hydrochloride.
Labetalol hydrochloride blunts the reflex tachycardia produced by nitroglycerin without preventing its hypotensive effect. If labetalol hydrochloride is used with nitroglycerin in patients with angina pectoris, additional antihypertensive effects may occur.
Care should be taken if labetalol is used concomitantly with calcium antagonists of the verapamil type.
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Risk of Anaphylactic Reaction
While taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
Labetalol hydrochloride tablets, USP are an adrenergic receptor blocking agent that has both selective alpha
1-adrenergic and nonselective beta-adrenergic receptor blocking actions in a single substance.
Labetalol hydrochloride, USP is a racemate, chemically designated as 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl] benzamide monohydrochloride, and it has the following structure:
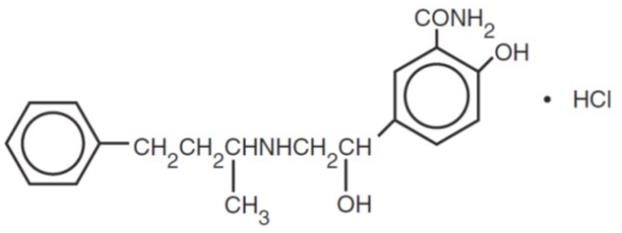
Labetalol hydrochloride, USP has the molecular formula C
19H
24N
2O
3•HCl and a molecular weight of 364.87. It has two asymmetric centers and therefore exists as a molecular complex of two diastereoisomeric pairs. Dilevalol, the R,R’ stereoisomer, makes up 25% of racemic labetalol.
Labetalol hydrochloride, USP is a white or off-white crystalline powder, soluble in water.
Labetalol hydrochloride tablets, USP, for oral administration, contain 100 mg, 200 mg or 300 mg labetalol hydrochloride, USP.
In addition, each 100 mg tablet contains the following inactive ingredients: anhydrous lactose, carnauba wax, hypromellose, magnesium stearate, polyethylene glycol, polysorbate 80, pregelatinized starch (corn), red iron oxide, titanium dioxide and yellow iron oxide.
In addition, each 200 mg tablet contains the following inactive ingredients: anhydrous lactose, carnauba wax, hypromellose, magnesium stearate, polydextrose, polyethylene glycol, pregelatinized starch (corn), titanium dioxide and triacetin.
In addition, each 300 mg tablet contains the following inactive ingredients: anhydrous lactose, carnauba wax, FD&C Blue #2, hypromellose, magnesium stearate, polyethylene glycol, polysorbate 80, pregelatinized starch (corn), titanium dioxide.