Lanreotide Acetate
Lanreotide Acetate Prescribing Information
Injection: 60 mg/0.2 mL, 90 mg/0.3 mL, and 120 mg/0.5 mL sterile, single-dose, prefilled syringes fitted with an automatic needle guard. The prefilled syringes contain a white to pale yellow, semi-solid formulation.
Lanreotide Injection is contraindicated in patients with history of a hypersensitivity to lanreotide. Allergic reactions (including angioedema and anaphylaxis) have been reported following administration of lanreotide
The following adverse reactions have been identified during post-approval use of Lanreotide Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions to Lanreotide Injection are discussed in greater detail in other sections of the labeling:
- Cholelithiasis and Complications of Cholelithiasis [see Warnings and Precautions ()]
5.1 Cholelithiasis and Complications of CholelithiasisLanreotide Injection may reduce gallbladder motility and lead to gallstone formation; therefore, patients may need to be monitored periodically
[see Adverse Reactions , Clinical Pharmacology ]. There have been postmarketing reports of cholelithiasis (gallstones) resulting in complications, including cholecystitis, cholangitis, and pancreatitis, and requiring cholecystectomy in patients taking Lanreotide Injection. If complications of cholelithiasis are suspected, discontinue Lanreotide Injection and treat appropriately. - Hyperglycemia and Hypoglycemia [see Warnings and Precautions ()]
5.2 Hyperglycemia and HypoglycemiaPharmacological studies in animals and humans show that lanreotide, like somatostatin and other somatostatin analogs, inhibits the secretion of insulin and glucagon. Hence, patients treated with Lanreotide Injection may experience hypoglycemia or hyperglycemia.
Blood glucose levels should be monitored when lanreotide treatment is initiated, or when the dose is altered, and antidiabetic treatment should be adjusted accordingly
[see Adverse Reactions ]. - Cardiovascular Abnormalities [see Warnings and Precautions ()]
5.3 Cardiovascular AbnormalitiesThe most common overall cardiac adverse reactions observed in three pooled Lanreotide Injection cardiac studies in patients with acromegaly were sinus bradycardia (12/217, 5.5%), bradycardia (6/217, 2.8%), and hypertension (12/217, 5.5%)
[see Adverse Reactions ].In 81 patients with baseline heart rates of 60 beats per minute (bpm) or greater treated with Lanreotide Injection in Study 3, the incidence of heart rate less than 60 bpm was 23% (19/81) as compared to 16% (15/94) of placebo treated patients; 10 patients (12%) had documented heart rates less than 60 bpm on more than one visit. The incidence of documented episodes of heart rate less than 50 bpm as well as the incidence of bradycardia reported as an adverse event was 1% in each treatment group.
Initiate appropriate medical management in patients who develop symptomatic bradycardia.In patients without underlying cardiac disease, Lanreotide Injection may lead to a decrease in heart rate without necessarily reaching the threshold of bradycardia. In patients suffering from cardiac disorders prior to Lanreotide Injection treatment, sinus bradycardia may occur. Care should be taken when initiating treatment with Lanreotide Injection in patients with bradycardia.
- Thyroid Function Abnormalities [see Warnings and Precautions ()]
5.4 Thyroid Function AbnormalitiesSlight decreases in thyroid function have been seen during treatment with lanreotide in acromegalic patients, though clinical hypothyroidism is rare (less than 1%). Thyroid function tests are recommended where clinically indicated.
- Steatorrhea and Malabsorption of Dietary Fats [see Warnings and Precautions ()]
5.6 Steatorrhea and Malabsorption of Dietary FatsNew onset steatorrhea, stool discoloration and loose stools have been reported in patients receiving somatostatin analogs, including lanreotide injection. Somatostatin analogs reversibly inhibit secretion of pancreatic enzymes and bile acids, which may result in malabsorption of dietary fats and subsequent symptoms of steatorrhea, loose stools, abdominal bloating, and weight loss. If new occurrence or worsening of these symptoms are reported in patients receiving Lanreotide Injection, evaluate patients for potential pancreatic exocrine insufficiency and manage accordingly.
Lanreotide Injection 60 mg/0.2 mL, 90 mg/0.3 mL, and 120 mg/0.5 mL is a prolonged-release formulation for deep subcutaneous injection. It contains the drug substance lanreotide acetate, a synthetic octapeptide with a biological activity similar to naturally occurring somatostatin, water for injection and acetic acid (for pH adjustment).
Lanreotide Injection is available as sterile, ready-to-use, single-dose prefilled syringes containing lanreotide acetate supersaturated bulk solution of 24.6% w/w lanreotide base.
Each syringe contains: | LANREOTIDE ACETATE | LANREOTIDE ACETATE | LANREOTIDE ACETATE |
60 mg/0.2 mL | 90 mg/0.3 mL | 120 mg/0.5 mL | |
Lanreotide acetate | 74.9 mg | 108.2 mg | 141.5 mg |
Acetic Acid | q.s. | q.s. | q.s. |
Water for injection | 196.8 mg | 284.5 mg | 372.1 mg |
Total Weight | 273.9 mg | 395.9 mg | 517.8 mg |
Lanreotide acetate is a synthetic cyclical octapeptide analog of the natural hormone, somatostatin. Lanreotide acetate is chemically known as [cyclo S-S]-3-(2-naphthyl)-D-alanyl-L-cysteinyl-L- tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-L-threoninamide, acetate salt. Its molecular weight is 1096.34 (base) and its amino acid sequence is:
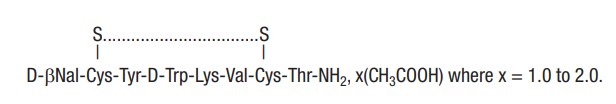
The Lanreotide Injection in the prefilled syringe is a white to pale yellow, semi-solid formulation.
Lanreotide Injection is supplied in strengths of 60 mg/0.2 mL, 90 mg/0.3 mL, and 120 mg/0.5 mL as a white to pale yellow, semi-solid formulation in a single, sterile, prefilled, ready-to-use, polypropylene syringe (fitted with an automatic needle guard) fitted with a 20 mm needle covered by a sheath.
Each prefilled syringe is sealed in a laminated pouch and packed in a carton.
NDC 69097-907-67 60 mg/0.2 mL, sterile, prefilled syringe
NDC 69097-908-67 90 mg/0.3 mL, sterile, prefilled syringe
NDC 69097-906-67 120 mg/0.5 mL, sterile, prefilled syringe
Store Lanreotide Injection in the refrigerator at 2°C to 8°C (36°F to 46°F). Protect from light.
Store in the original package.
IMPORTANT
If you have any questions about this medication or procedure, call 1-866- 604-3268.
2. Lanreotide Injection should be administered by a Healthcare Professional.
3. If this pre-filled syringe is dropped or damaged in any way, please contact
 | A. Storage of Lanreotide Injection When you receive the medication, follow these steps for storing Lanreotide Injection. Important: Lanreotide Injection MUST BE REFRIGERATED. DO NOT ALLOW IT TO REACH ROOM TEMPERATURE UNTIL READY TO USE. A1. Remove box from cold pack. Do not open box.A2. Check the following:a. Box does not look damaged b. Expiration date has not passed. c. Dose is as prescribed
Note: Call 1-866-604-3268 if you have any questions.A3. Place unopened box in your refrigerator. DO NOT PLACE IN FREEZER. |
 | B. Prepare to Inject B1. Confirm that date of this injection is as prescribed.B2. Remove box from refrigerator. Open box and remove contents.B3. Confirm that pouch is sealed and not damaged. |
 | B4. Check that the dose is as prescribed and the expiration date has not passed.B5. Let pouch sit for 30 MINUTES to reach room temperature. |
or
| DO NOT OPEN the pouch until ready to inject. Injection of cold medication may be painful. Note: Product left in its sealed pouch at room temperature (not to exceed 104° F or 40 °C) for up to 72 hours may be returned to the refrigerator for continued storage and use at a later time.B6. Find a clean, comfortable area for the patient to relax during procedure. It’s important that the patient remains as still as possible during the injection. |
 | B7. The person administering the injection must wash his/her hands with soap and water. Follow the doctor or institution’s policy on the use of surgical gloves during this procedure. |
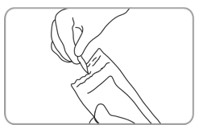 | B8. Tear open pouch along dotted line starting at the notch. |
 | |
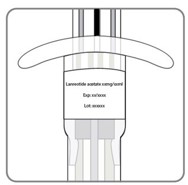 | B9. Do the following:a. Prior to administration, inspect the LANREOTIDE INJECTION syringe visually for particulate matter and discoloration. Do not administer if particulate matter or discoloration is observed. The content of the prefilled syringe is a semi-solid phase having a gel-like appearance, with viscous characteristics and a color varying from white to pale yellow. The supersaturated solution can also contain micro bubbles that can clear up during injection. These differences are normal and do not interfere with the quality of the product. b. Confirm that expiration date on the prefilled syringe has not passed. c. Make sure it is the right dosage:
d. Set device aside on the empty pouch. Note: Call 1-866-604-3268 if you have any questions. |
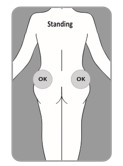
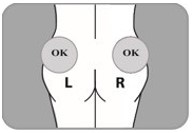 | B10. Choose which side of the buttocks to inject.
|
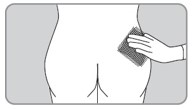
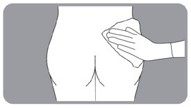 | B11. Clean area to be injected. |
C. Inject Important: This is a single-use pre-filled syringe with a retractable needle. ALL the medication must be injected during this use.If you drop or damage this pre-filled syringe in any way, please call 1-866-604-3268. Follow these injection instructions exactly! This procedure may be different from your past experience. | |
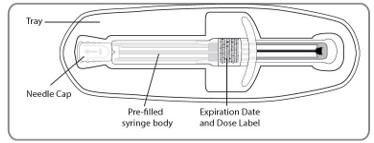
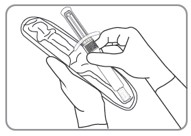 | C1. BEFORE injecting,REMOVE the pre-filled syringe from its tray. Discard tray. |
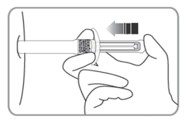
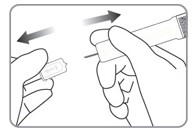 | C2. REMOVE NEEDLE CAP
|
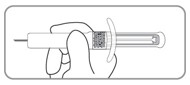 | C3. Hold pre-filled syringe by the syringe body. The pre-filled syringe is now ready for injection. |
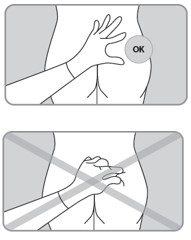 | C4. FLATTEN injection area using the thumb and index finger of your other hand to stretch the skin.DO NOT pinch skin. |
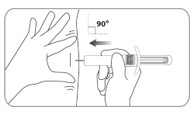 | C5. Insert needle PERPENDICULAR to the skin (90 degree angle).
|
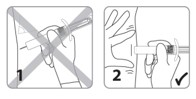 | 1. Do not insert needle at an acute angle. 2. Make sure needle is fully inserted. |
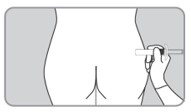 | C6. When needle is completely inserted, release injection site that has been flattened by your hand. |
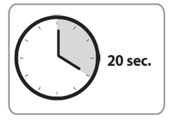
| C7. Push plunger with STEADY, VERY FIRM PRESSURE. • The medication is thicker and harder to push than you might expect. • While depressing plunger, slowly count to 20 and CONTINUE STEADY PRESSURE on the plunger. You may find it helpful to say:a. “1 one-thousand” b. “2 one-thousand” c. “3 one-thousand” up to “20 one thousand” Note: Pushing the plunger too fast may cause discomfort to the patient or may break device. |
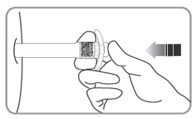 | C8. GIVE PLUNGER A FINAL PUSH to make sure you cannot depress the plunger further.Continue steady pressure with your thumb. |
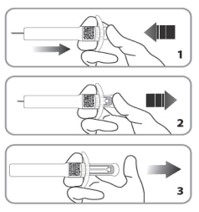 | C9. While continuing to hold down the plunger, remove the needle from the injection site (1), then allow the needle to retract by removing your thumb from the plunger (2) (3).If needle does not retract, push plunger again to engage safety mechanism. The needle will then retract. |
 | C10. If needed, gently apply a gauze pad to injection area.Important: NEVER RUB OR MASSAGE the Injection site. |
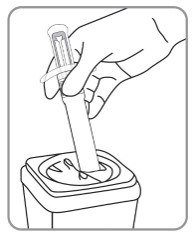 | D. Dispose of pre-filled syringe D1. Discard used pre-filled syringe into a hard plastic container with a screw top (such as a detergent bottle) or sharps container as per your institutional policy. |
 | D2. Wash your hands. |
Pharmathen International S.A.,
Rodopi, Greece
Cipla USA Inc.
10 Independence Boulevard, Suite 300
Warren, NJ 07059