Lapatinib Prescribing Information
Lapatinib tablets are indicated in combination with:
- capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress human epidermal growth factor receptor 2 (HER2) and who have received prior therapy, including an anthracycline, a taxane, and trastuzumab.Limitations of Use: Patients should have disease progression on trastuzumab prior to initiation of treatment with lapatinib tablets in combination with capecitabine.
- letrozole for the treatment of postmenopausal women with hormone receptor-positive metastatic breast cancer that overexpresses the HER2 receptor for whom hormonal therapy is indicated.
Lapatinib tablets in combination with an aromatase inhibitor has not been compared to a trastuzumab-containing chemotherapy regimen for the treatment of metastatic breast cancer.
Tablets: 250 mg - Each yellow, oval, film-coated tablet, debossed with “L250” on one side and “TV” on the other side contains 398 mg lapatinib ditosylate equivalent to 250 mg of lapatinib.
Lapatinib tablets are contraindicated in patients with known severe hypersensitivity (e.g., anaphylaxis) to this product or any of its components.
Lapatinib is a small molecule and a member of the 4-anilinoquinazoline class of kinase inhibitors. It is present as the monohydrate of the ditosylate salt, with chemical name
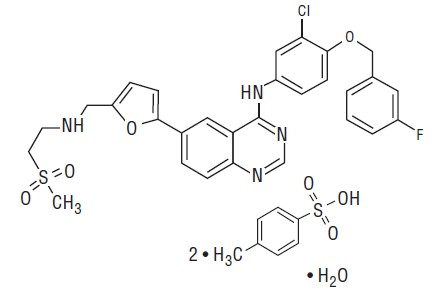
Lapatinib is a yellow solid, and its solubility in water is 0.007 mg/mL and in 0.1N HCl is 0.001 mg/mL at 25°C.
Each 250 mg tablet of lapatinib contains 405 mg of lapatinib ditosylate monohydrate, equivalent to 398 mg of lapatinib ditosylate or 250 mg lapatinib free base.
The inactive ingredients of lapatinib tablets are as follows: iron oxide yellow, magnesium stearate, microcrystalline cellulose, polyethylene glycol 3350, polyvinyl alcohol (part hydrolyzed), povidone, sodium starch glycolate Type A, talc, and titanium dioxide.
Lapatinib tablets are available as follows:
250 mg - Each yellow, oval, film-coated tablet, debossed with “L250” on one side and “TV” on the other side contains 398 mg lapatinib ditosylate equivalent to 250 mg of lapatinib. Tablets are available in bottles of 150 (NDC 0480-3237-51).
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Keep this and all medications out of the reach of children.