Lenalidomide Prescribing Information
Lenalidomide is a thalidomide analogue and is contraindicated for use during pregnancy. Thalidomide is a known human teratogen that causes life-threatening human birth defects or embryo-fetal death [
Lenalidomide (LEN a LID oh mide) Capsules
Because of the embryo-fetal risk [
Required components of the
- Prescribers must be certified with theLenalidomide REMSprogram by enrolling and complying with the REMS requirements.
- Patients must sign a Patient-Physician agreement form and comply with the REMS requirements. In particular, female patients of reproductive potential who are not pregnant must comply with the pregnancy testing and contraception requirements[see Use in Specific Populations ]and males must comply with contraception requirements[see Use in Specific Populations ].
- Pharmacies must be certified with theLenalidomide REMSprogram, must only dispense to patients who are authorized to receive lenalidomide capsules and comply with REMS requirements.
Further information about the
The recommended starting dose of lenalidomide capsule is 10 mg daily. Treatment is continued or modified based upon clinical and laboratory findings. Continue treatment until disease progression or unacceptable toxicity.
Patients who are dosed initially at 10 mg and who experience thrombocytopenia should have their dosage adjusted as follows:
If baseline is at least 100,000/mcL | |
When Platelets | Recommended Course |
| Fall below 50,000/mcL Return to at least 50,000/mcL | Interrupt lenalidomide capsule treatment Resume lenalidomide capsule at 5 mg daily |
If baseline is below 100,000/mcL | |
When Platelets | Recommended Course |
| Fall to 50% of the baseline value If baseline is at least 60,000/mcL and returns to at least 50,000/mcL If baseline is below 60,000/mcL and returns to at least 30,000/mcL | Interrupt lenalidomide capsule treatment Resume lenalidomide capsule at 5 mg daily Resume lenalidomide capsule at 5 mg daily |
When Platelets | Recommended Course |
| Fall below 30,000/mcL or below 50,000/mcL with platelet transfusions | Interrupt lenalidomide capsule treatment |
| Return to at least 30,000/mcL (without hemostatic failure) | Resume lenalidomide capsule at 5 mg daily |
When Platelets | Recommended Course |
| Fall below 30,000/mcL or below 50,000/mcL with platelet transfusions Return to at least 30,000/mcL (without hemostatic failure) | Interrupt lenalidomide capsule treatment Resume lenalidomide capsule at 2.5 mg daily |
If baseline ANC is at least 1,000/mcL | |
When Neutrophils | Recommended Course |
| Fall below 750/mcL Return to at least 1,000/mcL | Interrupt lenalidomide capsule treatment Resume lenalidomide capsule at 5 mg daily |
If baseline ANC is below 1,000/mcL | |
When Neutrophils | Recommended Course |
| Fall below 500/mcL Return to at least 500/mcL | Interrupt lenalidomide capsule treatment Resume lenalidomide capsule at 5 mg daily |
When Neutrophils | Recommended Course |
| Fall below 500/mcL for at least 7 days or below 500/mcL associated with fever (at least 38.5°C) Return to at least 500/mcL | Interrupt lenalidomide capsule treatment Resume lenalidomide capsule at 2.5 mg daily |
When Neutrophils | Recommended Course |
| Fall below 500/mcL for at least 7 days or below 500/mcL associated with fever (at least 38.5°C) Return to at least 500/mcL | Interrupt lenalidomide capsule treatment Resume lenalidomide capsule at 2.5 mg daily |
Warnings and Precautions (5.1, 5.2) 8/2021
Warnings and Precautions (5.1, 5.11) 5/2022
Lenalidomide is a thalidomide analogue indicated for the treatment of adult patients with:
- Multiple myeloma (MM), in combination with dexamethasone ().
1.1 Multiple MyelomaLenalidomide capsules in combination with dexamethasone are indicated for the treatment of adult patients with multiple myeloma (MM).
Lenalidomide capsules are indicated as maintenance therapy in adult patients with MM following autologous hematopoietic stem cell transplantation (auto-HSCT).
- MM, as maintenance following autologous hematopoietic stem cell transplantation (auto-HSCT) ().
1.1 Multiple MyelomaLenalidomide capsules in combination with dexamethasone are indicated for the treatment of adult patients with multiple myeloma (MM).
Lenalidomide capsules are indicated as maintenance therapy in adult patients with MM following autologous hematopoietic stem cell transplantation (auto-HSCT).
- Transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities ().
1.2 Myelodysplastic SyndromesLenalidomide capsules are indicated for the treatment of adult patients with transfusion-dependent anemia due to low-or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities.
- Mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib ().
1.3 Mantle Cell LymphomaLenalidomide capsules are indicated for the treatment of adult patients with mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib.
- Previously treated follicular lymphoma (FL), in combination with a rituximab product ().
1.4 Follicular LymphomaLenalidomide capsules in combination with a rituximab product, is indicated for the treatment of adult patients with previously treated follicular lymphoma (FL).
- Previously treated marginal zone lymphoma (MZL), in combination with a rituximab product ().
1.5 Marginal Zone LymphomaLenalidomide capsules in combination with a rituximab product, is indicated for the treatment of adult patients with previously treated marginal zone lymphoma (MZL).
- Lenalidomide capsules are not indicated and are not recommended for the treatment of patients with chronic lymphocytic leukemia (CLL) outside of controlled clinical trials.
- MM combination therapy: 25 mg once daily orally on Days 1-21 of repeated 28-day cycles. ().
2.1 Recommended Dosage for Multiple MyelomaLenalidomide Capsule Combination TherapyThe recommended starting dose of lenalidomide capsule is 25 mg orally once daily on Days 1-21 of repeated 28-day cycles in combination with dexamethasone. Refer to Section 14.1 for specific dexamethasone dosing. For patients greater than 75 years old, the starting dose of dexamethasone may be reduced
[see Clinical Studies ]. Treatment should be continued until disease progression or unacceptable toxicity.In patients who are not eligible for auto-HSCT, treatment should continue until disease progression or unacceptable toxicity. For patients who are auto-HSCT-eligible, hematopoietic stem cell mobilization should occur within 4 cycles of a lenalidomide capsule-containing therapy
[see Warnings and Precautions ].Dose Adjustments for Hematologic Toxicities During MM TreatmentDose modification guidelines, as summarized in Table 1 below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicity judged to be related to lenalidomide capsule.
v
Table 1: Dose Adjustments for Hematologic Toxicities for MM Platelet countsThrombocytopenia in MMWhen PlateletsRecommended CourseDays 1-21 of repeated 28-day cycleFall below 30,000/mcL Interrupt lenalidomide capsule treatment, follow CBC weekly Return to at least 30,000/mcL Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily For each subsequent drop below 30,000/mcL Interrupt lenalidomide capsule treatment Return to at least 30,000/mcL Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily Absolute Neutrophil counts (ANC)Neutropenia in MMWhen NeutrophilsRecommended CourseDays 1-21 of repeated 28-day cycleFall below 1,000/mcL Interrupt lenalidomide capsule treatment, follow CBC weekly Return to at least 1,000/mcL and neutropenia is the only toxicity Resume lenalidomide capsule at 25 mg daily or initial starting dose Return to at least 1,000/mcL and if other toxicity Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily For each subsequent drop below 1,000/mcL Interrupt lenalidomide capsule treatment Return to at least 1,000/mcL Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily Lenalidomide Capsule Maintenance Therapy Following Auto-HSCTFollowing auto-HSCT, initiate lenalidomide capsule maintenance therapy after adequate hematologic recovery (ANC at least 1,000/mcL and/or platelet counts at least 75,000/mcL). The recommended starting dose of lenalidomide capsule is 10 mg once daily continuously (Days 1-28 of repeated 28-day cycles) until disease progression or unacceptable toxicity. After 3 cycles of maintenance therapy, the dose can be increased to 15 mg once daily if tolerated.
Dose Adjustments for Hematologic Toxicities During MM TreatmentDose modification guidelines, as summarized in Table 2 below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicity judged to be related to lenalidomide capsule.
Table 2: Dose Adjustments for Hematologic Toxicities for MM Platelet counts Thrombocytopenia in MMWhen PlateletsRecommended CourseFall below 30,000/mcL
Return to at least 30,000/mcLInterrupt lenalidomide capsule treatment, follow CBC weekly
Resume lenalidomide capsule at next lower dose, continuously for Days 1-28 of repeated 28-day cycleIf at the 5 mg daily dose, For a subsequent drop below 30,000/mcL
Return to at least 30,000/mcLInterrupt lenalidomide capsule treatment. Do not dose below 5 mg daily for Day 1 to 21 of 28 day cycle
Resume lenalidomide capsule at 5 mg daily for Days 1 to 21of 28-day cycle. Do not dose below 5 mg daily for Day 1 to 21 of 28 day cycleAbsolute Neutrophil counts (ANC) Neutropenia in MMWhen NeutrophilsRecommended CourseFall below 500/mcL
Return to at least 500/mcLInterrupt lenalidomide capsule treatment, follow CBC weekly
Resume lenalidomide capsule at next lower dose, continuously for Days 1-28 of repeated 28-day cycleIf at 5 mg daily dose, For a subsequent drop below 500/mcL
Return to at least 500/mcLInterrupt lenalidomide capsule treatment. Do not dose below 5 mg daily for Days 1 to 21 of 28-day cycle
Resume lenalidomide capsule at 5 mg daily for Days 1 to 21 of 28-day cycle. Do not dose below 5 mg daily for Days 1 to 21 of 28-day cycle - MM maintenance therapy following auto-HSCT: 10 mg once daily continuously on Days 1-28 of repeated 28-day cycles ().
2.1 Recommended Dosage for Multiple MyelomaLenalidomide Capsule Combination TherapyThe recommended starting dose of lenalidomide capsule is 25 mg orally once daily on Days 1-21 of repeated 28-day cycles in combination with dexamethasone. Refer to Section 14.1 for specific dexamethasone dosing. For patients greater than 75 years old, the starting dose of dexamethasone may be reduced
[see Clinical Studies ]. Treatment should be continued until disease progression or unacceptable toxicity.In patients who are not eligible for auto-HSCT, treatment should continue until disease progression or unacceptable toxicity. For patients who are auto-HSCT-eligible, hematopoietic stem cell mobilization should occur within 4 cycles of a lenalidomide capsule-containing therapy
[see Warnings and Precautions ].Dose Adjustments for Hematologic Toxicities During MM TreatmentDose modification guidelines, as summarized in Table 1 below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicity judged to be related to lenalidomide capsule.
v
Table 1: Dose Adjustments for Hematologic Toxicities for MM Platelet countsThrombocytopenia in MMWhen PlateletsRecommended CourseDays 1-21 of repeated 28-day cycleFall below 30,000/mcL Interrupt lenalidomide capsule treatment, follow CBC weekly Return to at least 30,000/mcL Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily For each subsequent drop below 30,000/mcL Interrupt lenalidomide capsule treatment Return to at least 30,000/mcL Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily Absolute Neutrophil counts (ANC)Neutropenia in MMWhen NeutrophilsRecommended CourseDays 1-21 of repeated 28-day cycleFall below 1,000/mcL Interrupt lenalidomide capsule treatment, follow CBC weekly Return to at least 1,000/mcL and neutropenia is the only toxicity Resume lenalidomide capsule at 25 mg daily or initial starting dose Return to at least 1,000/mcL and if other toxicity Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily For each subsequent drop below 1,000/mcL Interrupt lenalidomide capsule treatment Return to at least 1,000/mcL Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily Lenalidomide Capsule Maintenance Therapy Following Auto-HSCTFollowing auto-HSCT, initiate lenalidomide capsule maintenance therapy after adequate hematologic recovery (ANC at least 1,000/mcL and/or platelet counts at least 75,000/mcL). The recommended starting dose of lenalidomide capsule is 10 mg once daily continuously (Days 1-28 of repeated 28-day cycles) until disease progression or unacceptable toxicity. After 3 cycles of maintenance therapy, the dose can be increased to 15 mg once daily if tolerated.
Dose Adjustments for Hematologic Toxicities During MM TreatmentDose modification guidelines, as summarized in Table 2 below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicity judged to be related to lenalidomide capsule.
Table 2: Dose Adjustments for Hematologic Toxicities for MM Platelet counts Thrombocytopenia in MMWhen PlateletsRecommended CourseFall below 30,000/mcL
Return to at least 30,000/mcLInterrupt lenalidomide capsule treatment, follow CBC weekly
Resume lenalidomide capsule at next lower dose, continuously for Days 1-28 of repeated 28-day cycleIf at the 5 mg daily dose, For a subsequent drop below 30,000/mcL
Return to at least 30,000/mcLInterrupt lenalidomide capsule treatment. Do not dose below 5 mg daily for Day 1 to 21 of 28 day cycle
Resume lenalidomide capsule at 5 mg daily for Days 1 to 21of 28-day cycle. Do not dose below 5 mg daily for Day 1 to 21 of 28 day cycleAbsolute Neutrophil counts (ANC) Neutropenia in MMWhen NeutrophilsRecommended CourseFall below 500/mcL
Return to at least 500/mcLInterrupt lenalidomide capsule treatment, follow CBC weekly
Resume lenalidomide capsule at next lower dose, continuously for Days 1-28 of repeated 28-day cycleIf at 5 mg daily dose, For a subsequent drop below 500/mcL
Return to at least 500/mcLInterrupt lenalidomide capsule treatment. Do not dose below 5 mg daily for Days 1 to 21 of 28-day cycle
Resume lenalidomide capsule at 5 mg daily for Days 1 to 21 of 28-day cycle. Do not dose below 5 mg daily for Days 1 to 21 of 28-day cycle - MDS: 10 mg once daily ().
2.2 Recommended Dosage for Myelodysplastic SyndromesThe recommended starting dose of lenalidomide capsule is 10 mg daily. Treatment is continued or modified based upon clinical and laboratory findings. Continue treatment until disease progression or unacceptable toxicity.
Dose Adjustments for Hematologic Toxicities During MDS TreatmentPatients who are dosed initially at 10 mg and who experience thrombocytopenia should have their dosage adjusted as follows:
Platelet counts If thrombocytopenia develops WITHIN 4 weeks of starting treatment at 10 mg daily in MDS If baseline is at least 100,000/mcLWhen PlateletsRecommended CourseFall below 50,000/mcL
Return to at least 50,000/mcLInterrupt lenalidomide capsule treatment
Resume lenalidomide capsule at 5 mg dailyIf baseline is below 100,000/mcLWhen PlateletsRecommended CourseFall to 50% of the baseline value
If baseline is at least 60,000/mcL and returns to at least 50,000/mcL
If baseline is below 60,000/mcL and returns to at least 30,000/mcLInterrupt lenalidomide capsule treatment
Resume lenalidomide capsule at 5 mg daily
Resume lenalidomide capsule at 5 mg dailyIf thrombocytopenia develops AFTER 4 weeks of starting treatment at 10 mg daily in MDS When PlateletsRecommended CourseFall below 30,000/mcL or below 50,000/mcL with platelet transfusions Interrupt lenalidomide capsule treatment Return to at least 30,000/mcL (without hemostatic failure) Resume lenalidomide capsule at 5 mg daily Patients who experience thrombocytopenia at 5 mg daily should have their dosage adjusted as follows: If thrombocytopenia develops during treatment at 5 mg daily in MDS When PlateletsRecommended CourseFall below 30,000/mcL or below 50,000/mcL with platelet transfusions
Return to at least 30,000/mcL (without hemostatic failure)Interrupt lenalidomide capsule treatment
Resume lenalidomide capsule at 2.5 mg dailyPatients who are dosed initially at 10 mg and experience neutropenia should have their dosage adjusted as follows: Absolute Neutrophil counts (ANC) If neutropenia develops WITHIN 4 weeks of starting treatment at 10 mg daily in MDS If baseline ANC is at least 1,000/mcLWhen NeutrophilsRecommended CourseFall below 750/mcL
Return to at least 1,000/mcLInterrupt lenalidomide capsule treatment
Resume lenalidomide capsule at 5 mg dailyIf baseline ANC is below 1,000/mcLWhen NeutrophilsRecommended CourseFall below 500/mcL
Return to at least 500/mcLInterrupt lenalidomide capsule treatment
Resume lenalidomide capsule at 5 mg dailyIf neutropenia develops AFTER 4 weeks of starting treatment at 10 mg daily in MDS When NeutrophilsRecommended CourseFall below 500/mcL for at least 7 days or below 500/mcL associated with fever (at least 38.5°C)
Return to at least 500/mcLInterrupt lenalidomide capsule treatment
Resume lenalidomide capsule at 2.5 mg dailyPatients who experience neutropenia at 5 mg daily should have their dosage adjusted as follows: If neutropenia develops during treatment at 5 mg daily in MDS When NeutrophilsRecommended CourseFall below 500/mcL for at least 7 days or below 500/mcL associated with fever (at least 38.5°C)
Return to at least 500/mcLInterrupt lenalidomide capsule treatment
Resume lenalidomide capsule at 2.5 mg daily - MCL: 25 mg once daily orally on Days 1-21 of repeated 28-day cycles ().
2.3 Recommended Dosage for Mantle Cell LymphomaThe recommended starting dose of lenalidomide capsule is 25 mg/day orally on Days 1-21 of repeated 28-day cycles for relapsed or refractory mantle cell lymphoma. Treatment should be continued until disease progression or unacceptable toxicity.
Treatment is continued, modified or discontinued based upon clinical and laboratory findings.
Dose Adjustments for Hematologic Toxicities During MCL TreatmentDose modification guidelines as summarized below are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicities considered to be related to lenalidomide capsule.
Platelet counts Thrombocytopenia during treatment in MCL When PlateletsRecommended CourseFall below 50,000/mcL Interrupt lenalidomide capsule treatment and follow CBC weekly Return to at least 50,000/mcL Resume lenalidomide capsule at 5 mg less than the previous dose. Do not dose below 5 mg daily Absolute Neutrophil counts (ANC) Neutropenia during treatment in MCL When NeutrophilsRecommended CourseFall below 1,000/mcL for at least 7 days
OR
Falls below 1,000/mcL with an associated temperature at least 38.5°C
OR
Falls below 500/mcLInterrupt lenalidomide capsule treatment and follow CBC weekly Return to at least 1,000/mcL Resume lenalidomide capsule at 5 mg less than the previous dose. Do not dose below 5 mg daily - FL or MZL: 20 mg once daily orally on Days 1-21 of repeated 28-day cycles for up to 12 cycles ().
2.4 Recommended Dosage for Follicular Lymphoma or Marginal Zone LymphomaThe recommended starting dose of lenalidomide capsule is 20 mg orally once daily on Days 1-21 of repeated 28-day cycles for up to 12 cycles of treatment in combination with a rituximab-product. Refer to Section 14.4 for specific rituximab dosing from the AUGMENT trial. For dose adjustments due to toxicity with rituximab, refer to the product prescribing information.
Dose Adjustments for Hematologic Toxicities during FL or MZL TreatmentDose modification guidelines, as summarized below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicity judged to be related to lenalidomide capsule.
Platelet counts Thrombocytopenia during treatment in FL or MZL When PlateletsRecommended CourseFall below 50,000/mcL Interrupt lenalidomide capsule treatment and follow CBC weekly. Return to at least 50,000/mcL If patient starting dose was 20 mg daily, resume lenalidomide capsule at 5 mg less than the previous dose. Do not dose below 5 mg daily.
If patient starting dose was 10 mg daily, resume at 5 mg less than previous dose. Do not dose below 2.5 mg daily.Absolute Neutrophil counts (ANC) Neutropenia during treatment in FL or MZL When NeutrophilsRecommended CourseFall below 1,000/mcL for at least 7 days
OR
Falls below 1,000/mcL with an associated temperature at least 38.5°C
OR
Falls below 500 /mcLInterrupt lenalidomide capsule treatment and follow CBC weekly. Return to at least 1,000/mcL If patient starting dose was 20 mg daily, resume lenalidomide capsule at 5 mg less than the previous dose. Do not dose below 5 mg daily.
If patient starting dose was 10 mg daily, resume at 5 mg less than previous dose. Do not dose below 2.5 mg daily. - Renal impairment: Adjust starting dose based on the creatinine clearance value ().
2.6 Recommended Dosage for Patients with Renal ImpairmentThe recommendations for dosing patients with renal impairment are shown in the following table[see Clinical Pharmacology ].Table 3: Dose Adjustments for Patients with Renal Impairment Renal Function(Cockcroft-Gault)Dose in Lenalidomide Capsule Combination Therapy for MM and MCLDose in Lenalidomide Capsule Combination Therapy for FL and MZLDose in Lenalidomide Capsule Maintenance Therapy Following Auto-HSCT for MM and for MDSCLcr 30 to 60 mL/min 10 mg once daily 10 mg once daily 5 mg once daily CLcr below 30 mL/min (not requiring dialysis) 15 mg every other day 5 mg once daily 2.5 mg once daily CLcr below 30 mL/min (requiring dialysis) 5 mg once daily. On dialysis days, administer the dose following dialysis. 5 mg once daily. On dialysis days, administer the dose following dialysis. 2.5 mg once daily. On dialysis days, administer the dose following dialysis. Lenalidomide Capsule Combination Therapy for MM: For CLcr of 30 to 60 mL/min, consider escalating the dose to 15 mg after 2 cycles if the patient tolerates the 10 mg dose of lenalidomide capsule without dose-limiting toxicity.Lenalidomide Capsule Maintenance Therapy Following Auto-HSCT for MM and for MCL and MDS:Base subsequent lenalidomide capsule dose increase or decrease on individual patient treatment tolerance[see Dosage and Administration ].Lenalidomide Capsule Combination Therapy for FL or for MZL:For patients with CLcr of 30 to 60 mL/min, after 2 cycles, the lenalidomide capsule dose may be increased to 15 mg orally if the patient has tolerated therapy. - For concomitant therapy doses, see Full Prescribing Information (,
2.1 Recommended Dosage for Multiple MyelomaLenalidomide Capsule Combination TherapyThe recommended starting dose of lenalidomide capsule is 25 mg orally once daily on Days 1-21 of repeated 28-day cycles in combination with dexamethasone. Refer to Section 14.1 for specific dexamethasone dosing. For patients greater than 75 years old, the starting dose of dexamethasone may be reduced
[see Clinical Studies ]. Treatment should be continued until disease progression or unacceptable toxicity.In patients who are not eligible for auto-HSCT, treatment should continue until disease progression or unacceptable toxicity. For patients who are auto-HSCT-eligible, hematopoietic stem cell mobilization should occur within 4 cycles of a lenalidomide capsule-containing therapy
[see Warnings and Precautions ].Dose Adjustments for Hematologic Toxicities During MM TreatmentDose modification guidelines, as summarized in Table 1 below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicity judged to be related to lenalidomide capsule.
v
Table 1: Dose Adjustments for Hematologic Toxicities for MM Platelet countsThrombocytopenia in MMWhen PlateletsRecommended CourseDays 1-21 of repeated 28-day cycleFall below 30,000/mcL Interrupt lenalidomide capsule treatment, follow CBC weekly Return to at least 30,000/mcL Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily For each subsequent drop below 30,000/mcL Interrupt lenalidomide capsule treatment Return to at least 30,000/mcL Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily Absolute Neutrophil counts (ANC)Neutropenia in MMWhen NeutrophilsRecommended CourseDays 1-21 of repeated 28-day cycleFall below 1,000/mcL Interrupt lenalidomide capsule treatment, follow CBC weekly Return to at least 1,000/mcL and neutropenia is the only toxicity Resume lenalidomide capsule at 25 mg daily or initial starting dose Return to at least 1,000/mcL and if other toxicity Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily For each subsequent drop below 1,000/mcL Interrupt lenalidomide capsule treatment Return to at least 1,000/mcL Resume lenalidomide capsule at next lower dose. Do not dose below 2.5 mg daily Lenalidomide Capsule Maintenance Therapy Following Auto-HSCTFollowing auto-HSCT, initiate lenalidomide capsule maintenance therapy after adequate hematologic recovery (ANC at least 1,000/mcL and/or platelet counts at least 75,000/mcL). The recommended starting dose of lenalidomide capsule is 10 mg once daily continuously (Days 1-28 of repeated 28-day cycles) until disease progression or unacceptable toxicity. After 3 cycles of maintenance therapy, the dose can be increased to 15 mg once daily if tolerated.
Dose Adjustments for Hematologic Toxicities During MM TreatmentDose modification guidelines, as summarized in Table 2 below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicity judged to be related to lenalidomide capsule.
Table 2: Dose Adjustments for Hematologic Toxicities for MM Platelet counts Thrombocytopenia in MMWhen PlateletsRecommended CourseFall below 30,000/mcL
Return to at least 30,000/mcLInterrupt lenalidomide capsule treatment, follow CBC weekly
Resume lenalidomide capsule at next lower dose, continuously for Days 1-28 of repeated 28-day cycleIf at the 5 mg daily dose, For a subsequent drop below 30,000/mcL
Return to at least 30,000/mcLInterrupt lenalidomide capsule treatment. Do not dose below 5 mg daily for Day 1 to 21 of 28 day cycle
Resume lenalidomide capsule at 5 mg daily for Days 1 to 21of 28-day cycle. Do not dose below 5 mg daily for Day 1 to 21 of 28 day cycleAbsolute Neutrophil counts (ANC) Neutropenia in MMWhen NeutrophilsRecommended CourseFall below 500/mcL
Return to at least 500/mcLInterrupt lenalidomide capsule treatment, follow CBC weekly
Resume lenalidomide capsule at next lower dose, continuously for Days 1-28 of repeated 28-day cycleIf at 5 mg daily dose, For a subsequent drop below 500/mcL
Return to at least 500/mcLInterrupt lenalidomide capsule treatment. Do not dose below 5 mg daily for Days 1 to 21 of 28-day cycle
Resume lenalidomide capsule at 5 mg daily for Days 1 to 21 of 28-day cycle. Do not dose below 5 mg daily for Days 1 to 21 of 28-day cycle,2.4 Recommended Dosage for Follicular Lymphoma or Marginal Zone LymphomaThe recommended starting dose of lenalidomide capsule is 20 mg orally once daily on Days 1-21 of repeated 28-day cycles for up to 12 cycles of treatment in combination with a rituximab-product. Refer to Section 14.4 for specific rituximab dosing from the AUGMENT trial. For dose adjustments due to toxicity with rituximab, refer to the product prescribing information.
Dose Adjustments for Hematologic Toxicities during FL or MZL TreatmentDose modification guidelines, as summarized below, are recommended to manage Grade 3 or 4 neutropenia or thrombocytopenia or other Grade 3 or 4 toxicity judged to be related to lenalidomide capsule.
Platelet counts Thrombocytopenia during treatment in FL or MZL When PlateletsRecommended CourseFall below 50,000/mcL Interrupt lenalidomide capsule treatment and follow CBC weekly. Return to at least 50,000/mcL If patient starting dose was 20 mg daily, resume lenalidomide capsule at 5 mg less than the previous dose. Do not dose below 5 mg daily.
If patient starting dose was 10 mg daily, resume at 5 mg less than previous dose. Do not dose below 2.5 mg daily.Absolute Neutrophil counts (ANC) Neutropenia during treatment in FL or MZL When NeutrophilsRecommended CourseFall below 1,000/mcL for at least 7 days
OR
Falls below 1,000/mcL with an associated temperature at least 38.5°C
OR
Falls below 500 /mcLInterrupt lenalidomide capsule treatment and follow CBC weekly. Return to at least 1,000/mcL If patient starting dose was 20 mg daily, resume lenalidomide capsule at 5 mg less than the previous dose. Do not dose below 5 mg daily.
If patient starting dose was 10 mg daily, resume at 5 mg less than previous dose. Do not dose below 2.5 mg daily.,14.1 Multiple MyelomaRandomized, Open-Label Clinical Trial in Patients with Newly Diagnosed MM:A randomized multicenter, open-label, 3-arm trial of 1,623 patients, was conducted to compare the efficacy and safety of lenalidomide capsule and low-dose dexamethasone (Rd) given for 2 different durations of time to that of melphalan, prednisone and thalidomide (MPT) in newly diagnosed MM patients who were not a candidate for stem cell transplant. In the first arm of the study, Rd was given continuously until progressive disease [Arm Rd Continuous]. In the second arm, Rd was given for up to eighteen 28-day cycles [72 weeks, Arm Rd18]). In the third arm, melphalan, prednisone and thalidomide (MPT) was given for a maximum of twelve 42-day cycles (72 weeks). For the purposes of this study, a patient who was < 65 years of age was not a candidate for SCT if the patient refused to undergo SCT therapy or the patient did not have access to SCT due to cost or other reasons. Patients were stratified at randomization by age (≤75 versus >75 years), stage (ISS Stages I and II versus Stage III), and country.
Patients in the Rd Continuous and Rd18 arms received lenalidomide capsule 25 mg once daily on Days 1 to 21 of 28-day cycles. Dexamethasone was dosed 40 mg once daily on Days 1, 8, 15, and 22 of each 28-day cycle. For patients over > 75 years old, the starting dose of dexamethasone was 20 mg orally once daily on days 1,8,15, and 22 of repeated 28-day cycles. Initial dose and regimens for Rd Continuous and Rd18 were adjusted according to age and renal function. All patients received prophylactic anticoagulation with the most commonly used being aspirin.
The demographics and disease-related baseline characteristics of the patients were balanced among the 3 arms. In general, study subjects had advanced-stage disease. Of the total study population, the median age was 73 in the 3 arms with 35% of total patients > 75 years of age; 59% had ISS Stage I/II; 41% had ISS stage III; 9% had severe renal impairment (creatinine clearance [CLcr] < 30 mL/min); 23% had moderate renal impairment (CLcr > 30 to 50 mL/min; 44% had mild renal impairment (CLcr > 50 to 80 mL/min). For ECOG Performance Status, 29% were Grade 0, 49% Grade 1, 21% Grade 2, 0.4% ≥ Grade 3.
The primary efficacy endpoint, progression-free survival (PFS), was defined as the time from randomization to the first documentation of disease progression as determined by Independent Response Adjudication Committee (IRAC), based on International Myeloma Working Group [IMWG] criteria or death due to any cause, whichever occurred first during the study until the end of the PFS follow-up phase. For the efficacy analysis of all endpoints, the primary comparison was between Rd Continuous and MPT arms. The efficacy results are summarized in the table below. PFS was significantly longer with Rd Continuous than MPT: HR 0.72 (95% CI: 0.61-0.85 p <0.0001). A lower percentage of subjects in the Rd Continuous arm compared with the MPT arm had PFS events (52% versus 61%, respectively). The improvement in median PFS time in the Rd Continuous arm compared with the MPT arm was 4.3 months. The myeloma response rate was higher with Rd Continuous compared with MPT (75.1% versus 62.3%); with a complete response in 15.1% of Rd Continuous arm patients versus 9.3% in the MPT arm. The median time to first response was 1.8 months in the Rd Continuous arm versus 2.8 months in the MPT arm.
For the interim OS analysis with 03 March 2014 data cutoff, the median follow-up time for all surviving patients is 45.5 months, with 697 death events, representing 78% of prespecified events required for the planned final OS analysis (697/896 of the final OS events). The observed OS HR was 0.75 for Rd Continuous versus MPT (95% CI = 0.62, 0.90).
Table 13: Overview of Efficacy Results – Study MM-020 (Intent-to-treat Population) CR = complete response; d = low-dose dexamethasone; HR = hazard ratio; IRAC = Independent Response Adjudication Committee; M = melphalan; NE = not estimable; OS = overall survival; P = prednisone; PFS = progression-free survival; PR = partial response; R = lenalidomide capsule; Rd Continuous = Rd given until documentation of progressive disease; Rd18 = Rd given for ≤ 18 cycles; T = thalidomide; VGPR = very good partial response; vs= versus.
aThe median is based on the Kaplan-Meier estimate.
bThe 95% Confidence Interval (CI) about the median.
cBased on Cox proportional hazards model comparing the hazard functions associated with the indicated treatment arms.
dThe p-value is based on the unstratified log-rank test of Kaplan-Meier curve differences between the indicated treatment arms.
eBest assessment of response during the treatment phase of the study.
fIncluding patients with no response assessment data or whose only assessment was "response not evaluable."
gData cutoff date = 24 May 2013.
hData cutoff date = 3 March 2014.
Rd Continuous(N = 535)Rd18(N = 541)MPT(N = 547)PFS – IRAC (months)gNumber of PFS events 278 (52) 348 (64.3) 334 (61.1) MedianaPFS time, months (95% CI)b 25.5 (20.7, 29.4) 20.7 (19.4, 22) 21.2 (19.3, 23.2) HR [95% CI]c; p-valued Rd Continuous vs MPT 0.72 (0.61, 0.85); <0.0001 Rd Continuous vs Rd18 0.70 (0.60, 0.82) Rd18 vs MPT 1.03 (0.89, 1.20) Overall Survival (months)hNumber of Death events 208 (38.9) 228 (42.1) 261 (47.7) MedianaOS time, months (95% CI)b 58.9 (56, NE)f 56.7 (50.1, NE) 48.5 (44.2, 52 ) HR [95% CI]c Rd Continuous vs MPT 0.75 (0.62, 0.90) Rd Continuous vs Rd18 0.91 (0.75, 1.09) Rd18 vs MPT 0.83 (0.69, 0.99) Response Ratee– IRAC, n (%)gCR 81 (15.1) 77 (14.2) 51 (9.3) VGPR 152 (28.4) 154 (28.5) 103 (18.8) PR 169 (31.6) 166 (30.7) 187 (34.2) Overall response: CR, VGPR, or PR 402 (75.1) 397 (73.4) 341 (62.3) Kaplan-Meier Curves of Progression-free Survival Based on IRAC Assessment (ITT MM Population) Between Arms Rd Continuous, Rd18 and MPT Cutoff date: 24 May 2013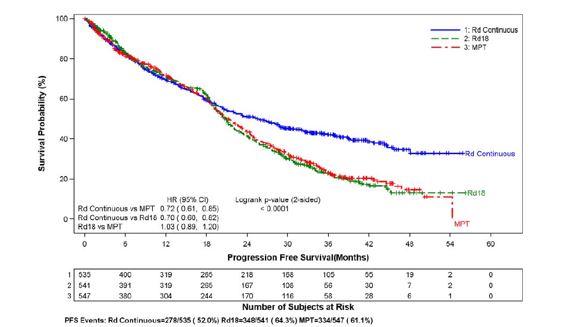
image CI = confidence interval; d = low-dose dexamethasone; HR = hazard ratio; IRAC = Independent Response Adjudication Committee; M = melphalan; P = prednisone; R = lenalidomide capsule; Rd Continuous = Rd given until documentation of progressive disease; Rd18 = Rd given for ≤ 18 cycles; T = thalidomide.
Kaplan-Meier Curves of Overall Survival (ITT MM Population) Between Arms Rd Continuous, Rd18 and MPT Cutoff date: 03 Mar 2014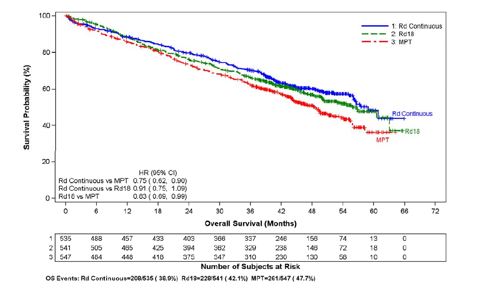
image CI = confidence interval; d = low-dose dexamethasone; HR = hazard ratio; M = melphalan; P = prednisone; R = lenalidomide capsule; Rd Continuous = Rd given until documentation of progressive disease; Rd18 = Rd given for ≤18 cycles; T = thalidomide.
Randomized, Placebo-Controlled Clinical Trials - Maintenance Following Auto-HSCT:Two multicenter, randomized, double-blind, parallel group, placebo-controlled studies were conducted to evaluate the efficacy and safety of lenalidomide capsule maintenance therapy in the treatment of MM patients after auto-HSCT. In Maintenance Study 1, patients between 18 and 70 years of age who had undergone induction therapy followed by auto-HSCT were eligible. Induction therapy must have occurred within 12 months. Within 90-100 days after auto-HSCT, patients with at least a stable disease response were randomized 1:1 to receive either lenalidomide capsule or placebo maintenance. In Maintenance Study 2, patients aged < 65 years at diagnosis who had undergone induction therapy followed by auto-HSCT and had achieved at least a stable disease response at the time of hematologic recovery were eligible. Within 6 months after auto-HSCT, patients were randomized 1:1 to receive either lenalidomide capsule or placebo maintenance. Patients eligible for both trials had to have CLcr ≥30 mL/minute.
In both studies, the lenalidomide capsule maintenance dose was 10 mg once daily on days 1-28 of repeated 28-day cycles, could be increased to 15 mg once daily after 3 months in the absence of dose-limiting toxicity, and treatment was to be continued until disease progression or patient withdrawal for another reason. The dose was reduced, or treatment was temporarily interrupted or stopped, as needed to manage toxicity. A dose increase to 15 mg once daily occurred in 135 patients (58%) in Maintenance Study 1, and in 185 patients (60%) in Maintenance Study 2.
The demographics and disease-related baseline characteristics of the patients were similar across the two studies and reflected a typical MM population after auto-HSCT (see Table 14).
Table 14: Baseline Demographic and Disease-Related Characteristics – MM Maintenance Studies 1 and 2 Data cutoff date = 1 March 2015.
Maintenance Study 1Maintenance Study 2Lenalidomide Capsule
N = 231PlaceboN = 229Lenalidomide Capsule
N = 307PlaceboN = 307Age (years)Median 58 58 57.5 58.1 (Min, max) (29, 71) (39, 71) (22.7, 68.3) (32.3, 67) Sex, n (%)Male 121 (52) 129 (56) 169 (55) 181 (59) Female 110 (48) 100 (44) 138 (45) 126 (41) ISS Stage at Diagnosis, n (%)Stage I or II 120 (52) 131 (57) 232 (76) 250 (81) Stage I62 (27) 85 (37) 128 (42) 143 (47) Stage II58 (25) 46 (20) 104 (34) 107 (35) Stage III 39 (17) 35 (15) 66 (21) 46 (15) Missing 72 (31) 63 (28) 9 (3) 11 (4) CrCl at Post-auto-HSCT, n (%)< 50 mL/min 23 (10) 16 (7) 10 (3) 9 (3) ≥ 50 mL/min 201 (87) 204 (89) 178 (58) 200 (65) Missing 7 (3) 9 (4) 119 (39) 98 (32) The major efficacy endpoint of both studies was PFS defined from randomization to the date of progression or death, whichever occurred first; the individual studies were not powered for an overall survival endpoint. Both studies were unblinded upon the recommendations of their respective data monitoring committees and after surpassing the respective thresholds for preplanned interim analyses of PFS. After unblinding, patients continued to be followed as before. Patients in the placebo arm of Maintenance Study 1 were allowed to cross over to receive lenalidomide capsule before disease progression (76 patients [33%] crossed over to lenalidomide capsule); patients in Maintenance Study 2 were not recommended to cross over. The efficacy results are summarized in the following table. In both studies, the primary analysis of PFS at unblinding was significantly longer with lenalidomide capsule compared to placebo: Maintenance Study 1 HR 0.38 (95% CI: 0.27-0.54 p <0.001) and Maintenance Study 2 HR 0.50 (95% CI: 0.39-0.64 p <0.001). For both studies, PFS was updated with a cutoff date of 1 March 2015 as shown in the table and the following Kaplan Meier graphs. With longer follow-up (median 72.4 and 86.0 months, respectively), the updated PFS analyses for both studies continue to show a PFS advantage for lenalidomide capsule compared to placebo: Maintenance Study 1 HR 0.38 (95% CI: 0.28-0.50) with median PFS of 68.6 months and Maintenance Study 2 HR 0.53 (95% CI: 0.44-0.64) with median PFS of 46.3 months.
Descriptive analysis of OS data with a cutoff date of 1 February 2016 are provided in Table 15. Median follow-up time was 81.6 and 96.7 months for Maintenance Study 1 and Maintenance Study 2, respectively. Median OS was 111.0 and 84.2 months for lenalidomide capsule and placebo, respectively, for Maintenance Study 1, and 105.9 and 88.1 months, for lenalidomide capsule and placebo, respectively, for Maintenance Study 2.
Table 15: Progression-free Survival and Overall Survival from Randomization in MM Maintenance Studies 1 and 2 (ITT Post-Auto-HSCT Population) Date of Unblinding in Maintenance Study 1 and 2 = 17 December 2009 and 7 July 2010, respectively.
Auto-HSCT = autologous hematopoietic stem cell transplantation; CI = confidence interval; ITT = intent to treat; NE = not estimable; PFS = progression-free survival.
PFS at time of unblinding for Maintenance Study 2 was based on assessment by an Independent Review Committee. All other PFS analyses were based on assessment by investigator.
Note: The median is based on Kaplan-Meier estimate, with 95% CIs about the median overall PFS time. Hazard ratio is based on a proportional hazards model stratified by stratification factors comparing the hazard functions associated with treatment arms (lenalidomide capsule: placebo).
Maintenance Study 1Maintenance Study 2Lenalidomide CapsuleN = 231PlaceboN = 229Lenalidomide CapsuleN = 307PlaceboN = 307PFS at UnblindingPFS Events n (%) 46 (20) 98 (43) 103 (34) 160 (52) Median in months [95% CI] 33.9
[NE, NE]19
[16.2, 25.6]41.2
[38.3, NE]23.0
[21.2, 28.0]Hazard Ratio [95% CI] 0.38
[0.27, 0.54]0.50
[0.39, 0.64]Log-rank Test p-value <0.001 <0.001 PFS at Updated Analysis 1 March 2015 (Studies 1 and 2)PFS Events n (%) 97 (42) 116 (51) 191 (62) 248 (81) Median in months [95% CI] 68.6
[52.8, NE]22.5
[18.8, 30.0]46.3
[40.1, 56.6]23.8
[21.0, 27.3]Hazard Ratio [95% CI] 0.38
[0.28, 0.50]0.53
[0.44, 0.64]OS at Updated Analysis 1 Feb 2016 (Studies 1 and 2)OS Events n (%) 82 (35) 114 (50) 143 (47) 160 (52) Median in months [95% CI] 111
[101.8, NE]84.2
[71.0, 102.7]105.9
[88.8, NE]88.1
[80.7, 108.4]Hazard Ratio [95% CI] 0.59
[0.44, 0.78]0.90
[0.72, 1.13]Kaplan-Meier Curves of Progression-free Survival from Randomization (ITT Post-Auto-HSCT Population) in MM Maintenance Study 1 between Lenalidomide Capsule and Placebo Arms (Updated Cutoff Date 1 March 2015)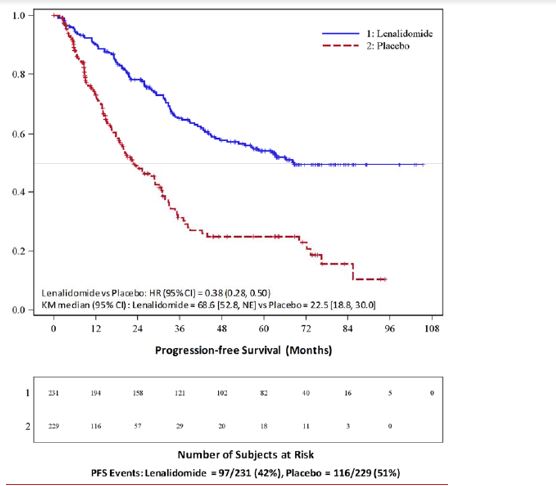
image Auto-HSCT = autologous hematopoietic stem cell transplantation; CI = confidence interval; HR = hazard ratio; ITT = intent to treat; KM = Kaplan-Meier; PFS = progression-free survival; vs = versus.
Kaplan-Meier Curves of Progression-free Survival from Randomization (ITT Post-Auto-HSCT Population) in MM Maintenance Study 2 between Lenalidomide Capsule and Placebo Arms (Updated Cutoff Date 1 March 2015)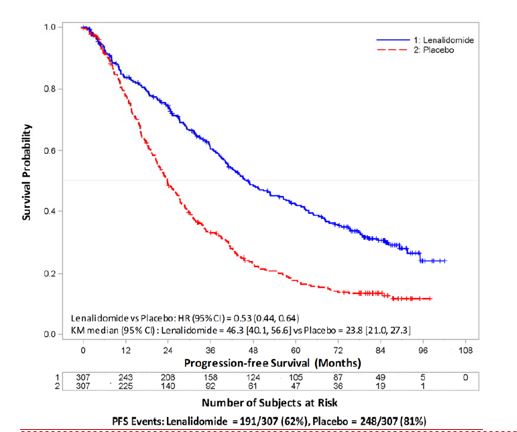
image Auto-HSCT = autologous hematopoietic stem cell transplantation; CI = confidence interval; HR = hazard ratio; ITT = intent to treat; KM = Kaplan-Meier; NE = not estimable; PFS = progression-free survival; vs = versus.
Randomized, Open-Label Clinical Studies in Patients with MMAfter At Least One Prior TherapyTwo randomized studies (Studies 1 and 2) were conducted to evaluate the efficacy and safety of lenalidomide. These multicenter, multinational, double-blind, placebo-controlled studies compared lenalidomide plus oral pulse high-dose dexamethasone therapy to dexamethasone therapy alone in patients with MM who had received at least one prior treatment. These studies enrolled patients with absolute neutrophil counts (ANC) ≥ 1000/mm3, platelet counts ≥ 75,000/mm3, serum creatinine ≤ 2.5 mg/dL, serum SGOT/AST or SGPT/ALT ≤ 3 x upper limit of normal (ULN), and serum direct bilirubin ≤ 2 mg/dL.
In both studies, patients in the lenalidomide/dexamethasone group took 25 mg of lenalidomide orally once daily on Days 1 to 21 and a matching placebo capsule once daily on Days 22 to 28 of each 28-day cycle. Patients in the placebo/dexamethasone group took 1 placebo capsule on Days 1 to 28 of each 28-day cycle. Patients in both treatment groups took 40 mg of dexamethasone orally once daily on Days 1 to 4, 9 to 12, and 17 to 20 of each 28-day cycle for the first 4 cycles of therapy.
The dose of dexamethasone was reduced to 40 mg orally once daily on Days 1 to 4 of each 28-day cycle after the first 4 cycles of therapy. In both studies, treatment was to continue until disease progression.
In both studies, dose adjustments were allowed based on clinical and laboratory findings. Sequential dose reductions to 15 mg daily, 10 mg daily and 5 mg daily were allowed for toxicity [
see Dosage and Administration].Table 16 summarizes the baseline patient and disease characteristics in the two studies. In both studies, baseline demographic and disease-related characteristics were comparable between the lenalidomide/dexamethasone and placebo/dexamethasone groups.
Table 16: Baseline Demographic and Disease-Related Characteristics – MM Studies 1 and 2 Study 1Study 2Lenalidomide/DexN=177Placebo/DexN=176Lenalidomide/DexN=176Placebo/DexN=175Patient CharacteristicsAge (years)
Median
Min, Max64
36, 8662
37, 8563
33, 8464
40, 82Sex
Male
Female106 (60%)
71 (40%)104 (59%)
72 (41%)104 (59%)
72 (41%)103 (59%)
72 (41%)Race/Ethnicity
White
Other141 (80%)
36 (20%)148 (84%)
28 (16%)172 (98%)
4 (2%)175 (100%)
0 (0%)ECOG Performance
Status 0-1157 (89%) 168 (95%) 150 (85%) 144 (82%) Disease CharacteristicsMultiple Myeloma Stage (Durie-Salmon)
I
II
III3%
32%
64%3%
31%
66%6%
28%
65%5%
33%
63%β2-microglobulin (mg/L)
≤ 2.5 mg/L
> 2.5 mg/L52 (29%)
125 (71%)51 (29%)
125 (71%)51 (29%)
125 (71%)48 (27%)
127 (73%)Number of Prior Therapies1
≥ 238%
62%38%
62%32%
68%33%
67%Types of Prior TherapiesStem Cell Transplantation 62% 61% 55% 54% Thalidomide 42% 46% 30% 38% Dexamethasone 81% 71% 66% 69% Bortezomib 11% 11% 5% 4% Melphalan 33% 31% 56% 52% Doxorubicin 55% 51% 56% 57% The primary efficacy endpoint in both studies was time to progression (TTP). TTP was defined as the time from randomization to the first occurrence of progressive disease.
Preplanned interim analyses of both studies showed that the combination of lenalidomide/dexamethasone was significantly superior to dexamethasone alone for TTP. The studies were unblinded to allow patients in the placebo/dexamethasone group to receive treatment with the lenalidomide/dexamethasone combination. For both studies, the extended follow-up survival data with crossovers were analyzed. In study 1, the median survival time was 39.4 months (95%CI: 32.9, 47.4) in lenalidomide/dexamethasone group and 31.6 months (95%CI: 24.1, 40.9) in placebo/dexamethasone group, with a hazard ratio of 0.79 (95% CI: 0.61-1.03). In study 2, the median survival time was 37.5 months (95%CI: 29.9, 46.6) in lenalidomide/dexamethasone group and 30.8 months (95%CI: 23.5, 40.3) in placebo/dexamethasone group, with a hazard ratio of 0.86 (95% CI: 0.65-1.14).
Table 17: TTP Results in MM Study 1 and Study 2 Study 1Study 2Lenalidomide/DexN=177Placebo/DexN=176Lenalidomide/DexN=176Placebo/Dex N=175TTPEvents n (%) 73 (41) 120 (68) 68 (39) 130 (74) Median TTP in months [95% CI] 13.9
[9.5, 18.5]4.7
[3.7, 4.9]12.1
[9.5, NE]4.7
[3.8, 4.8]Hazard Ratio
[95% CI]0.285
[0.210, 0.386]0.324
[0.240, 0.438]Log-rank Test p-value 3 <0.001 <0.001 ResponseComplete Response (CR) n (%) 23 (13) 1 (1) 27 (15) 7 (4) Partial Response (RR/PR) n (%) 84 (48) 33 (19) 77 (44) 34 (19) Overall Response n (%) 107 (61) 34 (19) 104 (59) 41 (23) p-value <0.001 <0.001 Odds Ratio [95% CI] 6.38
[3.95, 10.32]4.72
[2.98, 7.49]Kaplan-Meier Estimate of Time to Progression — MM Study 1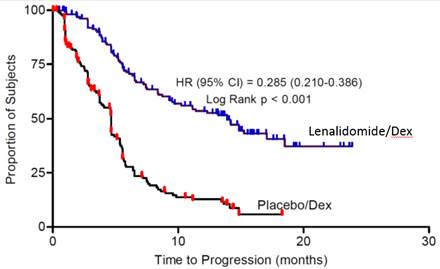 Kaplan-Meier Estimate of Time to Progression — MM Study 2
Kaplan-Meier Estimate of Time to Progression — MM Study 2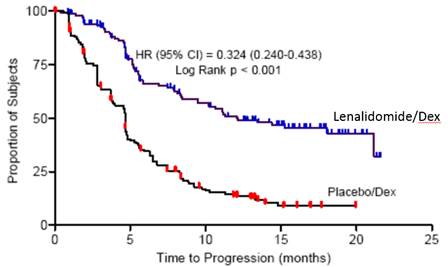 ).
).14.4 Follicular and Marginal Zone LymphomaThe efficacy of lenalidomide capsule with rituximab in patients with relapsed or refractory follicular and marginal zone lymphoma was evaluated in the AUGMENT (NCT01938001) and MAGNIFY (NCT01996865) trials.
AUGMENT is a randomized, double-blind, multicenter trial (n=358) in which patients with relapsed or refractory follicular or marginal zone lymphoma were randomized 1:1 to receive lenalidomide capsule and rituximab or rituximab and placebo. AUGMENT included patients diagnosed with Grade 1, 2, or 3a follicular lymphoma, who received at least 1 prior systemic therapy, were refractory or relapsed, not rituximab-refractory, had at least one measurable nodal or extranodal lesion by CT or MRI scan, and had adequate bone marrow, liver, and renal function. Randomization was stratified by follicular versus marginal zone lymphoma, previous rituximab therapy, and time since other anti-lymphoma therapy. In AUGMENT, lenalidomide capsule was administered orally 20 mg once daily for Days 1 to 21 of repeating 28-day cycles for a maximum of 12 cycles or until unacceptable toxicity. The dose of rituximab was 375 mg/m2every week in Cycle 1 (Days 1, 8, 15, and 22) and on Day 1 of every 28-day cycle from Cycles 2 through 5. All dosage calculations for rituximab were based on the patient's body surface area (BSA), using actual patient weight. Dose adjustments for lenalidomide capsule were allowed based on clinical and laboratory findings. A patient with moderate renal insufficiency (≥30 to <60 mL/minute) received a lower lenalidomide capsule starting dose of 10 mg daily on the same schedule. After 2 cycles, the lenalidomide capsule dose could be increased to 15 mg once daily on Days 1 to 21 of each 28-day cycle if the patient tolerated the medication.
MAGNIFY is an open-label, multicenter trial (n=232) in which patients with relapsed or refractory follicular, marginal zone, or mantle cell lymphoma received 12 induction cycles of lenalidomide capsule and rituximab. MAGNIFY included patients diagnosed with Grade 1, 2,3a, 3b follicular (including transformed), marginal zone, or mantle cell lymphoma Stage I to IV who were previously treated for their lymphoma, had been refractory or had a relapse after their last treatment, had at least one measurable nodal or extranodal lesion by CT or MRI scan, and had adequate bone marrow, liver, and renal function. Patients refractory to rituximab were also included. The information from the subjects who received at least 1 dose of initial therapy in the first 12 induction cycles (n=222) in the MAGNIFY trial was included in the evaluation of the efficacy of lenalidomide capsule/rituximab in patients with relapsed or refractory follicular and marginal zone lymphoma. In MAGNIFY, lenalidomide capsule 20 mg was given on Days 1-21 of repeated 28-day cycles for up to 12 cycles or until unacceptable toxicity, progression, or withdrawal of consent. The dose of rituximab was 375 mg/m2every week in Cycle 1 (Days 1, 8, 15, and 22) and on Day 1 of every other 28-day cycle (Cycles 3,5,7,9, and 11) up to 12 cycles therapy. All dosage calculations for rituximab were based on the patient BSA and actual weight. Dose adjustments were allowed based on clinical and laboratory findings.
The demographic and disease-related baseline characteristics in the AUGMENT and MAGNIFY trials are shown in the following table.
Table 21: Baseline Demographics and Disease-Related Characteristics of Patients with FL and MZL in AUGMENT and MAGNIFY Trials Data Cutoff: 22 June 2018 (AUGMENT) and 1 May 2017 (MAGNIFY).
aDefined by GELF criteria.
bPatient had either 0 (n=2) or 1 prior systemic therapy.
ECOG = Eastern Cooperative Oncology Group; FLIPI = follicular lymphoma international prognostic index
ParameterAUGMENT TrialMAGNIFY TrialLenalidomide Capsule+ Rituximab(N=178)Rituximab + Placebo(Control Arm)(N=180)Lenalidomide Capsule+ Rituximab(N=222)Age (years) Median (Max, Min) 64 (26, 86) 62 (35, 88) 65 (35, 91) Age distribution, n (%) <65 years 96 (54) 107 (59) 103 (46) ≥65 years 82 (46) 73 (41) 119 (54) Sex, n (%) Male 75 (42) 97 (54) 122 (55) Female 103 (58) 83 (46) 100 (45) Race White 118 (66) 115 (64) 206 (93) Other races 54 (30) 64 (36) 14 (6) Not collected or reported 6 (3) 1 (0.6) 2 (1) Body Surface Area (BSA, m2) Median (Max, Min) 1.8 (1.4, 3.1) 1.8 (1.3, 2.7) 2 (1.3, 2.6) Disease Type FL or MZL Follicular lymphoma 147 (83) 148 (82) 177 (80) Marginal zone lymphoma 31 (17) 32 (18) 45 (20) MZL subtype at diagnosis (investigator), n (%) MALT 14 (45) 16 (50) 10 (22) Nodal 8 (26) 10 (31) 25 (56) Splenic 9 (29) 6 (19) 10 (22) FL stage at diagnosis (investigator), n (%) FL Grade 1-2 125 (85) 123 (83) 149 (84) FL Grade 3a 22 (15) 25 (17) 28 (16) FLIPI score at baseline (calculated), n (%) Not Collected Low risk (0,1) 52 (29) 67 (37) Intermediate risk (2) 55 (31) 58 (32) High risk (≥3) 69 (39) 54 (30) Missing 2 (1) 1 (0.6) ECOG score at baseline, n (%) 0 116 (65) 128 (71) 102 (46) 1 60 (34) 50 (28) 113 (51) 2 2 (1) 2 (1) 7 (3) High tumor burdenaat baseline, n (%) Yes 97 (54) 86 (48) 148 (67) No 81 (46) 94 (52) 74 (33) Number of prior systemic antilymphoma therapies 1 102 (57) 97 (54) 94 (42)b >1 76 (43) 83 (46) 128 (58) In AUGMENT, efficacy was established in the intent-to-treat (ITT) population based on progression-free survival by Independent Review Committee using modified 2007 International Working Group response criteria. Efficacy results are summarized in Table 22.
Table 22: Efficacy Results for Patients in the AUGMENT Trial (ITT FL and MZL Population) aMedian estimate is from Kaplan-Meier analysis.
bhazard ratio and its CI were estimated from Cox proportional hazard model adjusting for the stratification 3: previous rituximab treatment (yes, no), time since last antilymphoma therapy (≤ 2, > 2 years), and disease histology (FL, MZL).
cp-value from log-rank test stratified by 3 factors noted above: previous rituximab treatment (yes, no), time since last antilymphoma therapy (≤ 2, > 2 years), and disease histology (FL, MZL).
dExact confidence interval for binomial distribution.
ParameterLenalidomide Capsule+ Rituximab(N=178)Rituximab + Placebo (N=180)PFSPatients with event, n (%) 68 (38.2) 115 (63.9) Death 6 (8.8) 2 (1.7) Progression of disease 62 (91.2) 113 (98.3) PFS, mediana[95% CI] (months) 39.4 [ 22.9, NE] 14.1 [11.4, 16.7] HR b[95% CI]0.46 [0.34, 0.62] p-valuec <0.0001 Objective response (CR+PR) , n(%) [95% CI]d138 (77.5) [70.7, 83.4] 96 (53.3) [45.8, 60.8] Kaplan-Meier Curves of Progression-free Survival by IRC Assessment Between Arms in AUGMENT Trial (ITT FL and MZL Population)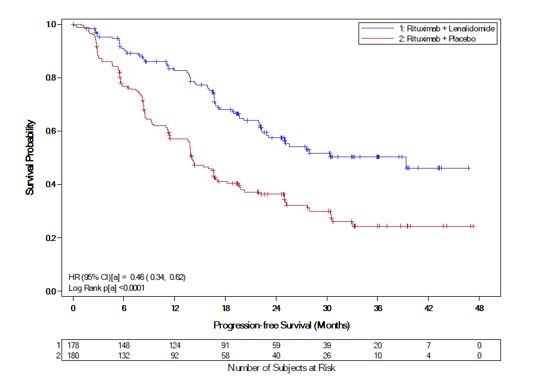
image a= Stratification factors included: previous rituximab treatment (y/n), time since last anti-lymphoma therapy (≤2 years, >2years), and disease histology (FL or MZL). CI = confidence interval; HR = hazard ratio; KM = Kaplan-Meier; PFS = progression-free survival
Follicular LymphomaIn AUGMENT, the objective response by IRC assessment for patients with follicular lymphoma was 80% (118/147) [95% CI: 73%, 86%]) in lenalidomide capsule with rituximab arm compared to 55% (82/148) [95% CI: 47, 64] in control arm.
In MAGNIFY, the overall response by investigator assessment was 59% (104/177) [95% CI: 51, 66] for patients with follicular lymphoma. Median duration of response was not reached with a median follow-up time of 7.9 months [95% CI: 4.6, 9.2].
Marginal Zone LymphomaIn AUGMENT, the objective response by IRC assessment for patients with marginal zone lymphoma was 65% (20/31) [95% CI: 45%, 81%] in lenalidomide capsule with rituximab arm compared to 44% (14/32) [95% CI: 26%, 62%] in control arm.
In MAGNIFY, the overall response by investigator assessment was 51% (23/45) [95% CI: 36, 66] for patients with marginal zone lymphoma. Median duration of response was not reached with a median follow-up time of 11.5 months [95% CI: 8.0, 18.9].
Capsules:
- 5 mg: White to off white powder filled in size '2' hard gelatin White and White capsule printed in black ink with 'Cipla 5 mg' on the cap and '381' on the body.
- 10 mg: White to off white powder filled in size '0' hard gelatin Blue green and Pale yellow capsule printed in black ink with 'Cipla 10 mg' on the cap and '382' on the body.
- 15 mg: White to off white powder filled in size '0' hard gelatin pale blue and white capsule printed in black ink with 'Cipla 15 mg' on the cap and '383' on the body.
- 20 mg: White to off white powder filled in size '0' hard gelatin Blue green and Pale blue capsule printed in black ink with 'Cipla 20 mg' on the cap and '384' on the body.
- 25 mg: White to off white powder filled in size '0' hard gelatin white and white capsule printed in black ink with 'Cipla 25 mg 'on the cap and '385' on the body.
- Lactation: Advise not to breastfeed. ().
8.2 LactationRisk SummaryThere is no information regarding the presence of lenalidomide in human milk, the effects of lenalidomide on the breastfed child, or the effects of lenalidomide on milk production. Because many drugs are excreted in human milk and because of the potential for adverse reactions in breastfed children from lenalidomide, advise women not to breastfeed during treatment with lenalidomide capsules.