Leuprolide Acetate - Leuprolide Acetate
(Leuprolide Acetate)Leuprolide Acetate - Leuprolide Acetate Prescribing Information
Leuprolide acetate injection is indicated in the palliative treatment of advanced prostatic cancer.
The recommended dose is 1 mg (0.2 mL or 20 unit mark) administered as a single daily subcutaneous injection. As with other drugs administered chronically by subcutaneous injection, the injection site should be varied periodically. Each 0.2 mL contains 1 mg of leuprolide acetate, sodium chloride for tonicity adjustment, 1.8 mg of benzyl alcohol as preservative and water for injection. The pH may have been adjusted with sodium hydroxide and/or acetic acid.
Follow the pictorial directions on the reverse side of this package insert for administration.
NOTE: As with all parenteral products, inspect the solution for discoloration and particulate matter before each use.
Leuprolide acetate injection is contraindicated in patients known to be hypersensitive to GnRH, GnRH agonist analogs or any of the excipients in leuprolide acetate injection: Reports of anaphylactic reactions to GnRH agonist analogs have been reported in the medical literature.
In the majority of patients testosterone levels increased above baseline during the first week, declining thereafter to baseline levels or below by the end of the second week of treatment. This transient increase was occasionally associated with a temporary worsening of signs and symptoms, usually manifested by an increase in bone pain (see
Initially, leuprolide acetate, like other LH-RH agonists, causes increases in serum levels of testosterone. Transient worsening of symptoms, or the occurrence of additional signs and symptoms of prostate cancer, may occasionally develop during the first few weeks of leuprolide acetate treatment. A small number of patients may experience a temporary increase in bone pain, which can be managed symptomatically. As with other LH-RH agonists, isolated cases of ureteral obstruction and spinal cord compression have been observed, which may contribute to paralysis with or without fatal complications.
Safe use of leuprolide acetate in pregnancy has not been established clinically. Leuprolide acetate may cause fetal harm.
Periodic monitoring of serum testosterone and prostate-specific antigen (PSA) levels is recommended, especially if the anticipated clinical or biochemical response to treatment has not been achieved. It should be noted that results of testosterone determinations are dependent on assay methodology. It is advisable to be aware of the type and precision of the assay methodology to make appropriate clinical and therapeutic decisions.
Potential exacerbations of signs and symptoms during the first few weeks of treatment is a concern in patients with vertebral metastases and/or urinary obstruction which, if aggravated, may lead to neurological problems or increase the obstruction.
In a comparative trial of leuprolide acetate injection versus DES, in 5% or more of the patients receiving either drug, the following adverse reactions were reported to have a possible or probable relationship to drug as ascribed by the treating physician. Often, causality is difficult to assess in patients with metastatic prostate cancer. Reactions considered not drug related are excluded.
* Physiologic effect of decreased testosterone. | ||
Leuprolide Acetate (N=98) | DES (N=101) | |
Number of Reports | ||
| Cardiovascular System | ||
| Congestive heart failure | 1 | 5 |
| ECG changes/ischemia | 19 | 22 |
| High blood pressure | 8 | 5 |
| Murmur | 3 | 8 |
| Peripheral edema | 12 | 30 |
| Phlebitis/thrombosis | 2 | 10 |
| Gastrointestinal System | ||
| Anorexia | 6 | 5 |
| Constipation | 7 | 9 |
| Nausea/vomiting | 5 | 17 |
| Endocrine System | ||
| *Decreased testicular size | 7 | 11 |
| *Gynecomastia/breast tenderness or pain | 7 | 63 |
| *Hot flashes | 55 | 12 |
| *Impotence | 4 | 12 |
| Hemic and Lymphatic System | ||
| Anemia | 5 | 5 |
| Musculoskeletal System | ||
| Bone pain | 5 | 2 |
| Myalgia | 3 | 9 |
| Central/Peripheral Nervous System | ||
| Dizziness/lightheadedness | 5 | 7 |
| General pain | 13 | 13 |
| Headache | 7 | 4 |
| Insomnia/sleep disorders | 7 | 5 |
| Respiratory System | ||
| Dyspnea | 2 | 8 |
| Sinus congestion | 5 | 6 |
| Integumentary System | ||
| Dermatitis | 5 | 8 |
| Urogenital System | ||
| Frequency/urgency | 6 | 8 |
| Hematuria | 6 | 4 |
| Urinary tract infection | 3 | 7 |
| Miscellaneous | ||
| Asthenia | 10 | 10 |
In this same study, the following adverse reactions were reported in less than 5% of the patients on leuprolide acetate.
In an additional clinical trial and from long-term observation of both studies, the following additional adverse events (excluding those considered not drug related) were reported for patients receiving leuprolide acetate.
See
Bioavailability by subcutaneous administration is comparable to that by intravenous administration.
The mean steady-state volume of distribution of leuprolide following intravenous bolus administration to healthy male volunteers was 27 L.
In healthy male volunteers, a 1 mg bolus of leuprolide administered intravenously revealed that the mean systemic clearance was 7.6 L/h, with a terminal elimination half-life of approximately 3 hours based on a two compartment model. In rats and dogs, administration of14C-labeled leuprolide was shown to be metabolized to smaller inactive peptides, a pentapeptide (Metabolite I), tripeptides (Metabolites II and III) and a dipeptide (Metabolite IV). These fragments may be further catabolized.
The major metabolite (M-I) plasma concentrations measured in 5 prostate cancer patients reached maximum concentration 2 to 6 hours after dosing and were approximately 6% of the peak parent drug concentration. One week after dosing, mean plasma M-I concentrations were approximately 20% of mean leuprolide concentrations.
Following administration of LUPRON DEPOT 3.75 mg to 3 patients, less than 5% of the dose was recovered as parent and M-I metabolite in the urine.
The pharmacokinetics of the drug in hepatically and renally impaired patients has not been determined.
No pharmacokinetic-based drug-drug interaction studies have been conducted with leuprolide acetate. However, because leuprolide acetate is a peptide that is primarily degraded by peptidase and not by cytochrome P-450 enzymes as noted in specific studies, and the drug is only about 46% bound to plasma proteins, drug interactions would not be expected to occur.
Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin releasing hormone (GnRH or LH-RH). The analog possesses greater potency than the natural hormone. The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) with the following structural formula:
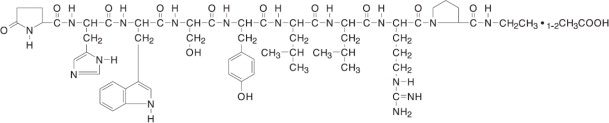
Leuprolide acetate injection is a sterile, aqueous solution intended for subcutaneous injection. It is available in a 2.8 mL multiple-dose vial containing leuprolide acetate (5 mg per mL), sodium chloride, USP (6.3 mg per mL) for tonicity adjustment, benzyl alcohol, NF as a preservative (9 mg per mL), and water for injection, USP. The pH may have been adjusted with sodium hydroxide, NF and/or acetic acid, NF. The pH range is 4.0 to 6.0.