Levofloxacin Prescribing Information
- Fluoroquinolones, including Levofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together[, including:]
5.1 Disabling and Potentially Irreversible Serious Adverse Reactions Including Tendinitis and Tendon Rupture, Peripheral Neuropathy, and Central Nervous System EffectsFluoroquinolones, including Levofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions can occur within hours to weeks after starting Levofloxacin. Patients of any age or without pre-existing risk factors have experienced these adverse reactions [
see Warnings and Precautions].Discontinue Levofloxacin immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including Levofloxacin, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
- Tendinitis and tendon rupture[]
5.2 Tendinitis and Tendon RuptureFluoroquinolones, including Levofloxacin, have been associated with an increased risk of tendinitis and tendon rupture in all ages
[see Warnings and Precautions (5.1)and Adverse Reactions (6.2)].This adverse reaction most frequently involves the Achilles tendon and has also been reported with the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendon sites. Tendinitis or tendon rupture can occur within hours or days of starting Levofloxacin or as long as several months after completion of fluoroquinolone therapy. Tendinitis and tendon rupture can occur bilaterally.The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is increased in patients over 60 years of age, in those taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Other factors that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have been reported in patients taking fluoroquinolones who do not have the above risk factors. Discontinue Levofloxacin immediately if the patient experiences pain, swelling, inflammation or rupture of a tendon. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug. Avoid Levofloxacin in patients who have a history of tendon disorders or tendon rupture
[see Adverse Reactions (6.3); Patient Counseling Information (17)].
- Peripheral neuropathy[]
5.3 Peripheral NeuropathyFluoroquinolones, including Levofloxacin, have been associated with an increased risk of peripheral neuropathy. Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving fluoroquinolones, including Levofloxacin. Symptoms may occur soon after initiation of Levofloxacin and may be irreversible in some patients
[see Warnings and Precautions (5.1)and Adverse Reactions (6.1, 6.2)].Discontinue Levofloxacin immediately if the patient experiences symptoms of neuropathy including pain, burning, tingling, numbness, and/or weakness or other alterations of sensation including light touch, pain, temperature, position sense, and vibratory sensation. Avoid fluoroquinolones, including Levofloxacin, in patients who have previously experienced peripheral neuropathy
[see Adverse Reactions (6), Patient Counseling Information (17)].
- Central nervous system effects[]
5.4 Central Nervous System EffectsPsychiatric Adverse Reactions
Fluoroquinolones, including levofloxacin, have been associated with an increased risk of psychiatric adverse reactions, including: toxic psychoses, hallucinations, or paranoia; depression, or suicidal thoughts; anxiety, agitation, restlessness, or nervousness; confusion, delirium, disorientation, or disturbances in attention; insomnia or nightmares; memory impairment. Attempted or completed suicide have been reported, especially in patients with a medical history of depression, or an underlying risk factor for depression. These reactions may occur following the first dose. If these reactions occur in patients receiving levofloxacin, discontinue levofloxacin and institute appropriate measures.
Central Nervous System Adverse Reactions of Seizures, Increased Intracranial Pressure, and Tremors
Fluoroquinolones, including levofloxacin, have been associated with an increased risk of seizures (convulsions), increased intracranial pressure (including pseudotumor cerebri), tremors, and lightheadedness. As with other fluoroquinolones, levofloxacin should be used with caution in patients with a known or suspected central nervous system (CNS) disorder that may predispose them to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose them to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction). If these reactions occur in patients receiving levofloxacin, discontinue levofloxacin and institute appropriate measures[see Adverse Reactions (6); Drug Interactions (7.4, 7.5); Patient Counseling Information (17)].
Fluoroquinolones, including Levofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions can occur within hours to weeks after starting Levofloxacin. Patients of any age or without pre-existing risk factors have experienced these adverse reactions [
Discontinue Levofloxacin immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including Levofloxacin, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
- Fluoroquinolones, including Levofloxacin, may exacerbate muscle weakness in patients with myasthenia gravis. Avoid Levofloxacin in patients with a known history of myasthenia gravis[.]
5.5 Exacerbation of Myasthenia GravisFluoroquinolones, including Levofloxacin, have neuromuscular blocking activity and may exacerbate muscle weakness in patients with myasthenia gravis. Postmarketing serious adverse reactions including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in patients with myasthenia gravis. Avoid Levofloxacin in patients with a known history of myasthenia gravis
[see Adverse Reactions (6.3); Patient Counseling Information (17)]. - Because fluoroquinolones, including Levofloxacin, have been associated with serious adverse reactions[see, reserve Levofloxacin for use in patients who have no alternative treatment options for the following indications:-
5.1 Disabling and Potentially Irreversible Serious Adverse Reactions Including Tendinitis and Tendon Rupture, Peripheral Neuropathy, and Central Nervous System EffectsFluoroquinolones, including Levofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions can occur within hours to weeks after starting Levofloxacin. Patients of any age or without pre-existing risk factors have experienced these adverse reactions [
see Warnings and Precautions].Discontinue Levofloxacin immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including Levofloxacin, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
)]5.15 Development of Drug Resistant BacteriaPrescribing Levofloxacin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria
[see Patient Counseling Information (17)].
- Uncomplicated urinary tract infection[]
1.12 Uncomplicated Urinary Tract InfectionsLevofloxacin is indicated for the treatment of uncomplicated urinary tract infections (mild to moderate) due to Escherichia coli, Klebsiella pneumoniae, or Staphylococcus saprophyticus.
Because fluoroquinolones, including Levofloxacin have been associated with serious adverse reactions
[see Warnings and Precautions (5.1- 5.15 )]and for some patients uncomplicated urinary tract infection is self-limiting, reserve Levofloxacin for treatment of uncomplicated urinary tract infections in patients who have no alternative treatment options. - Acute bacterial exacerbation of chronic bronchitis[]
1.13 Acute Bacterial Exacerbation of Chronic BronchitisLevofloxacin is indicated for the treatment of acute bacterial exacerbation of chronic bronchitis (ABECB) due to methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis.
Because fluoroquinolones, including Levofloxacin have been associated with serious adverse reactions
[see Warnings and Precautions (5.1-5.15)]and for some patients ABECB is self-limiting, reserve Levofloxacin for treatment of ABECB in patients who have no alternative treatment options. - Acute bacterial sinusitis[.]
1.14 Acute Bacterial Sinusitis: 5-day and 10 to 14 day Treatment RegimensLevofloxacin is indicated for the treatment of acute bacterial sinusitis (ABS) due to Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis
[see Clinical Studies (14.4)].
Because fluoroquinolones, including Levofloxacin have been associated with serious adverse reactions[see Warnings and Precautions (5.1- 5.15)]and for some patients ABS is self-limiting, reserve Levofloxacin for treatment of ABS in patients who have no alternative treatment options.
Warnings and Precautions, Hypersensitivity Reactions (
Serious and occasionally fatal hypersensitivity and/or anaphylactic reactions
Warnings and Precautions, Risk of Aortic Aneurysm and Dissection (
Epidemiologic studies report an increased rate of aortic aneurysm
Levofloxacin Injection is indicated for the treatment of adults (≥18 years of age) with mild, moderate, and severe infections caused by susceptible isolates of the designated microorganisms in the conditions listed in this section. Levofloxacin Injection is indicated when intravenous administration offers a route of administration advantageous to the patient (e.g., patient cannot tolerate an oral dosage form).
• Dosage in patients with normal renal function (
The usual dose of Levofloxacin Injection is 250 mg or 500 mg administered by slow infusion over 60 minutes every 24 hours or 750 mg administered by slow infusion over 90 minutes every 24 hours, as indicated by infection and described in Table 1. These recommendations apply to patients with creatinine clearance ≥ 50 mL/min. For patients with creatinine clearance <50 mL/min, adjustments to the dosing regimen are required
Type of Infection* | Dosed Every 24 hours | Duration (days)† |
| Nosocomial Pneumonia | 750 mg | 7 to 14 |
| Community Acquired Pneumonia‡ | 500 mg | 7 to 14 |
| Community Acquired Pneumonia§ | 750 mg | 5 |
| Complicated Skin and Skin Structure Infections (SSSI) | 750 mg | 7 to 14 |
| Uncomplicated SSSI | 500 mg | 7 to 10 |
| Chronic Bacterial Prostatitis | 500 mg | 28 |
| Inhalational Anthrax (Post-Exposure), adult and pediatric patients > 50 kgÞ,ß Pediatric patients < 50 kg and ≥ 6 months of ageÞ,ß | 500 mg see Table 2 below (2.2) | 60ß 60ß |
| Plague, adult and pediatric patients > 50 kgà Pediatric patients < 50 kg and ≥ 6 months of age | 500 mg see Table 2 below (2.2) | 10 to 14 10 to 14 |
| Complicated Urinary Tract Infection (cUTI) or Acute Pyelonephritis (AP)¶ | 750 mg | 5 |
| Complicated Urinary Tract Infection (cUTI) or Acute Pyelonephritis (AP)# | 250 mg | 10 |
| Uncomplicated Urinary Tract Infection | 250 mg | 3 |
| Acute Bacterial Exacerbation of Chronic Bronchitis (ABECB) | 500 mg | 7 |
| Acute Bacterial Sinusitis (ABS) | 750 mg | 5 |
| 500 mg | 10 to 14 |
* Due to the designated pathogens
†Sequential therapy (intravenous to oral) may be instituted at the discretion of the physician.
‡Due to methicillin-susceptible
§Due to
¶ This regimen is indicated for cUTI due to
#This regimen is indicated for cUTI due to
Þ Drug administration should begin as soon as possible after suspected or confirmed exposure to aerosolized
ßThe safety of Levofloxacin in adults for durations of therapy beyond 28 days or in pediatric patients for durations beyond 14 days has not been studied. An increased incidence of musculoskeletal adverse events compared to controls has been observed in pediatric patients
àDrug administration should begin as soon as possible after suspected or confirmed exposure to Yersinia pestis. Higher doses of Levofloxacin typically used for treatment of pneumonia can be used for treatment of plague, if clinically indicated.
Type of Infection | Dosed Every 24 hours | Duration (days)† |
Nosocomial Pneumonia (Levofloxacin is indicated for the treatment of nosocomial pneumonia due to methicillin susceptible Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Escherichia coli, Klebsiella pneumoniae, Haemophilus influenzae, or Streptococcus pneumoniae . Adjunctive therapy should be used as clinically indicated. WherePseudomonas aeruginosa is a documented or presumptive pathogen, combination therapy with an anti-pseudomonal β-lactam is recommended[see Clinical Studies (14.1)]. | 750 mg | 7 to 14 |
Community Acquired Pneumonia (Levofloxacin is indicated for the treatment of community-acquired pneumonia due to methicillin- susceptible Staphylococcus aureus, Streptococcus pneumoniae (including multidrug- resistantStreptococcus pneumoniae [MDRSP]),Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydophila pneumoniae, Legionella pneumophila, orMycoplasma pneumoniae [see Dosage and Administration (2.1)and Clinical Studies (14.2)]. MDRSP isolates are isolates resistant to two or more of the following antibacterials: penicillin (MIC ≥2 mcg/mL), 2nd generation cephalosporins, e.g., cefuroxime, macrolides, tetracyclines and trimethoprim/sulfamethoxazole. | 500 mg | 7 to 14 |
Community Acquired Pneumonia (Levofloxacin is indicated for the treatment of community-acquired pneumonia due to Streptococcus pneumoniae (excluding multi-drug-resistant isolates [MDRSP]),Haemophilus influenzae, Haemophilus parainfluenzae, Mycoplasma pneumoniae, orChlamydophila pneumoniae [see Dosage and Administration (2.1)and Clinical Studies (14.3)]. | 750 mg | 5 |
Complicated Skin and Skin Structure Infections (SSSI) (Levofloxacin is indicated for the treatment of complicated skin and skin structure infections due to methicillin-susceptible Staphylococcus aureus, Enterococcus faecalis, Streptococcus pyogenes, orProteus mirabilis [see Clinical Studies (14.5)]. | 750 mg | 7 to 14 |
Uncomplicated SSSI (Levofloxacin is indicated for the treatment of uncomplicated skin and skin structure infections (mild to moderate) including abscesses, cellulitis, furuncles, impetigo, pyoderma, wound infections, due to methicillin-susceptible Staphylococcus aureus, orStreptococcus pyogenes . | 500 mg | 7 to 10 |
Chronic Bacterial Prostatitis (Levofloxacin is indicated for the treatment of chronic bacterial prostatitis due to Escherichia coli, Enterococcus faecalis, or methicillin-susceptibleStaphylococcus epidermidis [see Clinical Studies (14.6)]. | 500 mg | 28 |
Inhalational Anthrax (Post-Exposure) (Levofloxacin is indicated for inhalational anthrax (post-exposure) to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis . The effectiveness of Levofloxacin is based on plasma concentrations achieved in humans, a surrogate endpoint reasonably likely to predict clinical benefit. Levofloxacin has not been tested in humans for the post-exposure prevention of inhalation anthrax. The safety of Levofloxacin in adults for durations of therapy beyond 28 days or in pediatric patients for durations of therapy beyond 14 days has not been studied. Prolonged Levofloxacin therapy should only be used when the benefit outweighs the risk[see Dosage and Administration (2.1, 2.2) and Clinical Studies (14.9)]. Adults and Pediatric Patients > 50 kg Pediatric Patients < 50 kg and ≥ 6 months of age | 500 mg 8 mg/kg BID (not to exceed 250 mg/dose) | 60 60 |
Plague (Levofloxacin is indicated for treatment of plague, including pneumonic and septicemic plague, due to Yersinia pestis (Y. pestis) and prophylaxis for plague in adults and pediatric patients, 6 months of age and older. Efficacy studies of Levofloxacin could not be conducted in humans with plague for ethical and feasibility reasons. Therefore, approval of this indication was based on an efficacy study conducted in animals[see Dosage and Administration (2.1, 2.2) and Clinical Studies (14.10) ].Adults and Pediatric Patients > 50 kg Pediatric Patients < 50 kg and ≥ 6 months of age | 500 mg 8 mg/kg BID (not to exceed 250 mg/dose) | 10 to 14 10 to 14 |
Complicated Urinary Tract Infection (Levofloxacin is indicated for the treatment of complicated urinary tract infections due to Escherichia coli, Klebsiella pneumoniae , orProteus mirabilis [see Clinical Studies (14.7)]. Levofloxacin is indicated for the treatment of acute pyelonephritis caused by Escherichia coli, including cases with concurrent bacteremia [see Clinical Studies (14.7, 14.8)]. | 750 mg | 5 |
Complicated Urinary Tract Infection (Levofloxacin is indicated for the treatment of complicated urinary tract infections (mild to moderate) due to Enterococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, orPseudomonas aeruginosa [see Clinical Studies (14.8)]. Levofloxacin is indicated for the treatment of acute pyelonephritis caused by Escherichia coli, including cases with concurrent bacteremia [see Clinical Studies (14.7, 14.8)]. | 250 mg | 10 |
Uncomplicated Urinary Tract Infection (Levofloxacin is indicated for the treatment of uncomplicated urinary tract infections (mild to moderate) due to Escherichia coli, Klebsiella pneumoniae, or Staphylococcus saprophyticus. Because fluoroquinolones, including Levofloxacin have been associated with serious adverse reactions [see Warnings and Precautions (5.1- 5.15 )] and for some patients uncomplicated urinary tract infection is self-limiting, reserve Levofloxacin for treatment of uncomplicated urinary tract infections in patients who have no alternative treatment options. | 250 mg | 3 |
Acute Bacterial Exacerbation of Chronic Bronchitis (Levofloxacin is indicated for the treatment of acute bacterial exacerbation of chronic bronchitis (ABECB) due to methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis. Because fluoroquinolones, including Levofloxacin have been associated with serious adverse reactions [see Warnings and Precautions (5.1-5.15)] and for some patients ABECB is self-limiting, reserve Levofloxacin for treatment of ABECB in patients who have no alternative treatment options. | 500 mg | 7 |
Acute Bacterial Sinusitis (Levofloxacin is indicated for the treatment of acute bacterial sinusitis (ABS) due to Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis [see Clinical Studies (14.4)]. Because fluoroquinolones, including Levofloxacin have been associated with serious adverse reactions [see Warnings and Precautions (5.1- 5.15)] and for some patients ABS is self-limiting, reserve Levofloxacin for treatment of ABS in patients who have no alternative treatment options. | 750 mg | 5 |
| 500 mg | 10 to 14 |
• Adjust dose for creatinine clearance < 50 mL/min (
Administer Levofloxacin with caution in the presence of renal insufficiency. Careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy since elimination of levofloxacin may be reduced.
No adjustment is necessary for patients with a creatinine clearance ≥ 50 mL/min.
In patients with impaired renal function (creatinine clearance <50 mL/min), adjustment of the dosage regimen is necessary to avoid the accumulation of levofloxacin due to decreased clearance
Table 3 shows how to adjust dose based on creatinine clearance.
Dosage in Normal Renal Function Every 24 hours | CreatinineClearance 20 to 49 mL/min | Creatinine Clearance 10 to 19 mL/min | Hemodialysis or Chronic Ambulatory Peritoneal Dialysis (CAPD) |
| 750 mg | 750 mg every 48 hours | 750 mg initial dose, then 500 mg every 48 hours | 750 mg initial dose, then 500 mg every 48 hours |
| 500 mg | 500 mg initial dose, then 250 mg every 24 hours | 500 mg initial dose, then 250 mg every 48 hours | 500 mg initial dose, then 250 mg every 48 hours |
| 250 mg | No dosage adjustment required | 250 mg every 48 hours. If treating uncomplicated UTI, then no dosage adjustment is required | No information on dosing adjustment is available |
Clearance of levofloxacin is substantially reduced and plasma elimination half-life is substantially prolonged in patients with impaired renal function (creatinine clearance < 50 mL/min), requiring dosage adjustment in such patients to avoid accumulation. Neither hemodialysis nor continuous ambulatory peritoneal dialysis (CAPD) is effective in removal of levofloxacin from the body, indicating that supplemental doses of Levofloxacin are not required following hemodialysis or CAPD
The mean ± SD pharmacokinetic parameters of levofloxacin determined under single and steady-state conditions following intravenous (IV) doses of Levofloxacin are summarized in Table 10.
Regimen | Cmax (mcg/mL) | Tmax (h) | AUC (mcg·h/mL) | CL/F1 (mL/min) | Vd/F2 (L) | t1/2 (h) | CLR (mL/min) |
Single Dose | |||||||
| 250 mg oral tablet3 | 2.8 ± 0.4 | 1.6 ± 1.0 | 27.2 ± 3.9 | 156 ± 20 | ND | 7.3 ± 0.9 | 142 ± 21 |
| 500 mg oral tablet3* | 5.1 ± 0.8 | 1.3 ± 0.6 | 47.9 ± 6.8 | 178 ± 28 | ND | 6.3 ± 0.6 | 103 ± 30 |
| 500 mg oral solution12 | 5.8 ± 1.8 | 0.8 ± 0.7 | 47.8 ± 10.8 | 183 ± 40 | 112 ± 37.2 | 7.0 ± 1.4 | ND |
| 500 mg IV3 | 6.2 ± 1.0 | 1.0 ± 0.1 | 48.3 ± 5.4 | 175 ± 20 | 90 ± 11 | 6.4 ± 0.7 | 112 ± 25 |
| 750 mg oral tablet5* | 9.3 ± 1.6 | 1.6 ± 0.8 | 101 ± 20 | 129 ± 24 | 83 ± 17 | 7.5 ± 0.9 | ND |
| 750 mg IV5 | 11.5 ± 4.04 | ND | 110 ± 40 | 126 ± 39 | 75 ± 13 | 7.5 ± 1.6 | ND |
Multiple Dose | |||||||
| 500 mg every 24h oral tablet3 | 5.7 ± 1.4 | 1.1 ± 0.4 | 47.5 ± 6.7 | 175 ± 25 | 102 ± 22 | 7.6 ± 1.6 | 116 ± 31 |
| 500 mg every 24h IV3 | 6.4 ± 0.8 | ND | 54.6 ± 11.1 | 158 ± 29 | 91 ± 12 | 7.0 ± 0.8 | 99 ± 28 |
| 500 mg or 250 mg every 24h IV, patients with bacterial infection6 | 8.7± 4.07 | ND | 72.5 ± 51.27 | 154 ± 72 | 111 ± 58 | ND | ND |
| 750 mg every 24h IV5 | 12.1 ± 4.14 | ND | 108 ± 34 | 126 ± 37 | 80 ± 27 | 7.9 ± 1.9 | ND |
500 mg oral tablet single dose, effects of gender and age: | |||||||
| Male8 | 5.5 ± 1.1 | 1.2 ± 0.4 | 54.4 ± 18.9 | 166 ± 44 | 89 ± 13 | 7.5 ± 2.1 | 126 ± 38 |
| Female9 | 7.0 ± 1.6 | 1.7 ± 0.5 | 67.7 ± 24.2 | 136 ± 44 | 62 ± 16 | 6.1 ± 0.8 | 106 ± 40 |
| Young10 | 5.5 ± 1.0 | 1.5 ± 0.6 | 47.5 ± 9.8 | 182 ± 35 | 83 ± 18 | 6.0 ± 0.9 | 140 ± 33 |
| Elderly11 | 7.0 ± 1.6 | 1.4 ± 0.5 | 74.7 ± 23.3 | 121 ± 33 | 67 ± 19 | 7.6 ± 2.0 | 91 ± 29 |
500 mg oral single dose tablet, patients with renal insufficiency : | |||||||
| CLCR 50 to 80 mL/min | 7.5 ± 1.8 | 1.5 ± 0.5 | 95.6 ± 11.8 | 88 ± 10 | ND | 9.1 ± 0.9 | 57 ± 8 |
| CLCR 20 to 49 mL/min | 7.1 ± 3.1 | 2.1 ± 1.3 | 182.1 ± 62.6 | 51 ± 19 | ND | 27 ± 10 | 26 ± 13 |
| CLCR <20 mL/min | 8.2 ± 2.6 | 1.1 ± 1.0 | 263.5 ± 72.5 | 33 ± 8 | ND | 35 ± 5 | 13 ± 3 |
| Hemodialysis | 5.7 ± 1.0 | 2.8 ± 2.2 | ND | ND | ND | 76 ± 42 | ND |
| CAPD | 6.9 ± 2.3 | 1.4 ± 1.1 | ND | ND | ND | 51 ± 24 | ND |
1clearance/bioavailability
2volume of distribution/bioavailability
3healthy males 18 to 53 years of age
460 min infusion for 250 mg and 500 mg doses, 90 min infusion for 750 mg dose
5healthy male and female subjects 18 to 54 years of age
6500 mg every 48h for patients with moderate renal impairment (CLCR 20 to 50 mL/min) and infections of the respiratory tract or skin
7dose-normalized values (to 500 mg dose), estimated by population pharmacokinetic modeling
8healthy males 22 to 75 years of age
9healthy females 18 to 80 years of age
10young healthy male and female subjects 18 to 36 years of age
11healthy elderly male and female subjects 66 to 80 years of age
12healthy males and females 19 to 55 years of age.
* Absolute bioavailability; F=0.99 ± 0.08 from a 500 mg tablet and F=0.99 ± 0.06 from a 750 mg tablet;
ND=not determined.
Levofloxacin is rapidly and essentially completely absorbed after oral administration. Peak plasma concentrations are usually attained one to two hours after oral dosing. The absolute bioavailability of levofloxacin from a 500 mg tablet and 750 mg tablet of Levofloxacin are both approximately 99%, demonstrating complete oral absorption of levofloxacin. Following a single intravenous dose of Levofloxacin to healthy volunteers, the mean ± SD peak plasma concentration attained was 6.2 ± 1.0 mcg/mL after a 500 mg dose infused over 60 minutes and 11.5 ± 4.0 mcg/mL after a 750 mg dose infused over 90 minutes. Levofloxacin Oral Solution and Tablet formulations are bioequivalent.
Levofloxacin pharmacokinetics are linear and predictable after single and multiple oral or IV dosing regimens. Steady-state conditions are reached within 48 hours following a 500 mg or 750 mg once-daily dosage regimen. The mean ± SD peak and trough plasma concentrations attained following multiple once-daily oral dosage regimens were approximately 5.7 ± 1.4 and 0.5 ± 0.2 mcg/mL after the 500 mg doses, and 8.6 ± 1.9 and 1.1 ± 0.4 mcg/mL after the 750 mg doses, respectively. The mean ± SD peak and trough plasma concentrations attained following multiple once-daily IV regimens were approximately 6.4 ± 0.8 and 0.6 ± 0.2 mcg/mL after the 500 mg doses, and 12.1 ± 4.1 and 1.3 ± 0.71 mcg/mL after the 750 mg doses, respectively. Oral administration of a 500 mg dose of Levofloxacin with food prolongs the time to peak concentration by approximately 1 hour and decreases the peak concentration by approximately 14% following tablet and approximately 25% following oral solution administration. Therefore, Levofloxacin Tablets can be administered without regard to food. It is recommended that Levofloxacin Oral Solution be taken 1 hour before or 2 hours after eating.
The plasma concentration profile of levofloxacin after IV administration is similar and comparable in extent of exposure (AUC) to that observed for Levofloxacin Tablets when equal doses (mg/mg) are administered. Therefore, the oral and IV routes of administration can be considered interchangeable (see Figure 2 and Figure 3).
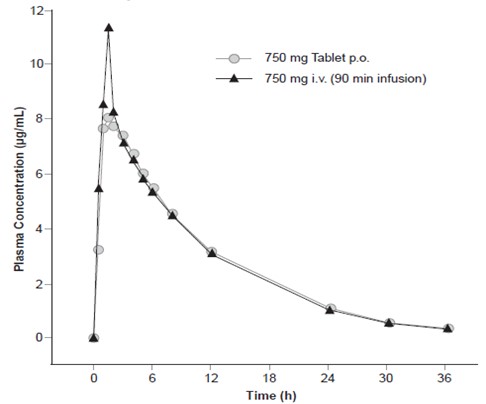
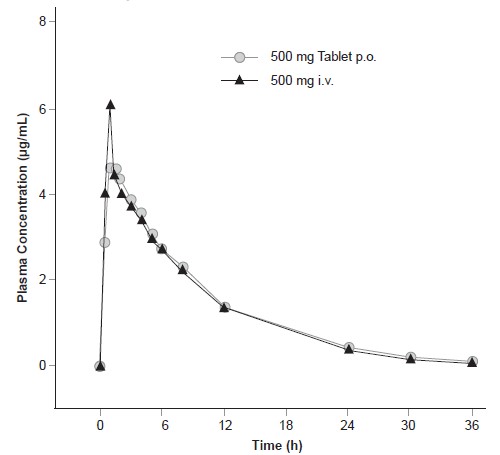
The mean volume of distribution of levofloxacin generally ranges from 74 to 112 L after single and multiple 500 mg or 750 mg doses, indicating widespread distribution into body tissues.
Levofloxacin reaches its peak levels in skin tissues and in blister fluid of healthy subjects at approximately 3 hours after dosing. The skin tissue biopsy to plasma AUC ratio is approximately 2 and the blister fluid to plasma AUC ratio is approximately 1 following multiple once-daily oral administration of 750 mg and 500 mg doses of Levofloxacin, respectively, to healthy subjects. Levofloxacin also penetrates well into lung tissues. Lung tissue concentrations were generally 2- to 5-fold higher than plasma concentrations and ranged from approximately 2.4 to 11.3 mcg/g over a 24-hour period after a single 500 mg oral dose.
Levofloxacin is stereochemically stable in plasma and urine and does not invert metabolically to its enantiomer, D-ofloxacin. Levofloxacin undergoes limited metabolism in humans and is primarily excreted as unchanged drug in the urine. Following oral administration, approximately 87% of an administered dose was recovered as unchanged drug in urine within 48 hours, whereas less than 4% of the dose was recovered in feces in 72 hours. Less than 5% of an administered dose was recovered in the urine as the desmethyl and N-oxide metabolites, the only metabolites identified in humans. These metabolites have little relevant pharmacological activity.
Levofloxacin is excreted largely as unchanged drug in the urine. The mean terminal plasma elimination half-life of levofloxacin ranges from approximately 6 to 8 hours following single or multiple doses of levofloxacin given orally or intravenously. The mean apparent total body clearance and renal clearance range from approximately 144 to 226 mL/min and 96 to 142 mL/min, respectively. Renal clearance in excess of the glomerular filtration rate suggests that tubular secretion of levofloxacin occurs in addition to its glomerular filtration. Concomitant administration of either cimetidine or probenecid results in approximately 24% and 35% reduction in the levofloxacin renal clearance, respectively, indicating that secretion of levofloxacin occurs in the renal proximal tubule. No levofloxacin crystals were found in any of the urine samples freshly collected from subjects receiving Levofloxacin.
There are no significant differences in levofloxacin pharmacokinetics between young and elderly subjects when the subjects’ differences in creatinine clearance are taken into consideration. Following a 500 mg oral dose of Levofloxacin to healthy elderly subjects (66 to 80 years of age), the mean terminal plasma elimination half-life of levofloxacin was about 7.6 hours, as compared to approximately 6 hours in younger adults. The difference was attributable to the variation in renal function status of the subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by age. Levofloxacin dose adjustment based on age alone is not necessary
The pharmacokinetics of levofloxacin following a single 7 mg/kg intravenous dose were investigated in pediatric patients ranging in age from 6 months to 16 years. Pediatric patients cleared levofloxacin faster than adult patients, resulting in lower plasma exposures than adults for a given mg/kg dose. Subsequent pharmacokinetic analyses predicted that a dosage regimen of 8 mg/kg every 12 hours (not to exceed 250 mg per dose) for pediatric patients 6 months to 17 years of age would achieve comparable steady state plasma exposures (AUC0-24and Cmax) to those observed in adult patients administered 500 mg of levofloxacin once every 24 hours.
There are no significant differences in levofloxacin pharmacokinetics between male and female subjects when subjects’ differences in creatinine clearance are taken into consideration. Following a 500 mg oral dose of Levofloxacin to healthy male subjects, the mean terminal plasma elimination half-life of levofloxacin was about 7.5 hours, as compared to approximately 6.1 hours in female subjects. This difference was attributable to the variation in renal function status of the male and female subjects and was not believed to be clinically significant. Drug absorption appears to be unaffected by the gender of the subjects. Dose adjustment based on gender alone is not necessary.
The effect of race on levofloxacin pharmacokinetics was examined through a covariate analysis performed on data from 72 subjects: 48 white and 24 non-white. The apparent total body clearance and apparent volume of distribution were not affected by the race of the subjects.
Clearance of levofloxacin is substantially reduced and plasma elimination half-life is substantially prolonged in adult patients with impaired renal function (creatinine clearance < 50 mL/min), requiring dosage adjustment in such patients to avoid accumulation. Neither hemodialysis nor continuous ambulatory peritoneal dialysis (CAPD) is effective in removal of levofloxacin from the body, indicating that supplemental doses of Levofloxacin are not required following hemodialysis or CAPD
Pharmacokinetic studies in hepatically impaired patients have not been conducted. Due to the limited extent of levofloxacin metabolism, the pharmacokinetics of levofloxacin are not expected to be affected by hepatic impairment
The pharmacokinetics of levofloxacin in patients with serious community-acquired bacterial infections are comparable to those observed in healthy subjects.
The potential for pharmacokinetic drug interactions between Levofloxacin and antacids, warfarin, theophylline, cyclosporine, digoxin, probenecid, and cimetidine has been evaluated


• IV Injection, Single-Dose: Slow IV infusion only, over 60 or 90 minutes depending on dose. Avoid rapid or bolus IV (
Caution: Rapid or bolus intravenous infusion of Levofloxacin has been associated with hypotension and must be avoided. Levofloxacin Injection should be infused intravenously slowly over a period of not less than 60 or 90 minutes, depending on the dosage. Levofloxacin Injection should be administered only by intravenous infusion. It is not for intramuscular, intrathecal, intraperitoneal, or subcutaneous administration.
Adequate hydration of patients receiving intravenous Levofloxacin should be maintained to prevent the formation of highly concentrated urine. Crystalluria and cylindruria have been reported with quinolones
• Dilute single-dose vials to 5 mg/mL prior to IV infusion (
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Because only limited data are available on the compatibility of Levofloxacin Injection with other intravenous substances, additives or other medications should not be added to Levofloxacin Injection in Single-Dose Vials, or infused simultaneously through the same intravenous line. If the same intravenous line is used for sequential infusion of several different drugs, the line should be flushed before and after infusion of Levofloxacin Injection with an infusion solution compatible with Levofloxacin Injection and with any other drug(s) administered via this common line.
Single-dose vials require dilution prior to administration.
Levofloxacin Injection is supplied in single-dose vials containing a concentrated levofloxacin solution with the equivalent of 500 mg (20 mL vial) and 750 mg (30 mL vial) of levofloxacin in Water for Injection, USP. The 20 mL and 30 mL vials each contain 25 mg of levofloxacin/mL. These Levofloxacin Injection single-dose vials must be further diluted with an appropriate solution prior to intravenous administration [
Compatible Intravenous Solutions: Any of the following intravenous solutions may be used to prepare a 5 mg/mL levofloxacin solution with the approximate pH values:
Intravenous Fluids | Final pH of Levofloxacin Solution |
| 0.9% Sodium Chloride Injection, USP | 4.71 |
| 5% Dextrose Injection, USP | 4.58 |
| 5% Dextrose/0.9% NaCl Injection | 4.62 |
| 5% Dextrose in Lactated Ringers | 4.92 |
| Plasma-Lyte® 56/5% Dextrose Injection | 5.03 |
| 5% Dextrose, 0.45% Sodium Chloride, and 0.15% Potassium Chloride Injection | 4.61 |
| Sodium Lactate Injection (M/6) | 5.54 |
Since no preservative or bacteriostatic agent is present in this product, aseptic technique must be used in preparation of the final intravenous solution. Since the vials are for single-dose only, any unused portion remaining in the vial should be discarded. When used to prepare two 250 mg doses from the 20 mL vial containing 500 mg of levofloxacin, the full content of the vial should be withdrawn at once using a single-entry procedure, and a second dose should be prepared and stored for subsequent use [see Stability of Levofloxacin Injection Following Dilution].
Prepare the desired dosage of levofloxacin according to the table below:
Desired Dosage Strength | From Appropriate Vial, Withdraw Volume | Volume of Diluent | Infusion Time |
| 250 mg | 10 mL (20 mL Vial) | 40 mL | 60 min |
| 500 mg | 20 mL (20 mL Vial) | 80 mL | 60 min |
| 750 mg | 30 mL (30 mL Vial) | 120 mL | 90 min |
For example, to prepare a 500 mg dose using the 20 mL vial (25 mg/mL), withdraw 20 mL and dilute with a compatible intravenous solution to a total volume of 100 mL.
This intravenous drug product should be inspected visually for particulate matter prior to administration. Samples containing visible particles should be discarded.
Stability of Levofloxacin Injection Following Dilution: Levofloxacin Injection, when diluted in a compatible intravenous fluid to a concentration of 5 mg/mL, is stable for 72 hours when stored at or below 25°C (77°F) and for 14 days when stored under refrigeration at 5°C (41°F) in plastic intravenous containers. Solutions that are diluted in a compatible intravenous solution and frozen in glass bottles or plastic intravenous containers are stable for 6 months when stored at -20°C (-4°F). Thaw frozen solutions at room temperature 25°C (77°F) or in a refrigerator 8°C (46°F). Do not force thaw by microwave irradiation or water bath immersion. Do not refreeze after initial thawing.
• Do not mix with other medications in vial or IV line (
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Because only limited data are available on the compatibility of Levofloxacin Injection with other intravenous substances, additives or other medications should not be added to Levofloxacin Injection in Single-Dose Vials, or infused simultaneously through the same intravenous line. If the same intravenous line is used for sequential infusion of several different drugs, the line should be flushed before and after infusion of Levofloxacin Injection with an infusion solution compatible with Levofloxacin Injection and with any other drug(s) administered via this common line.
Single-dose vials require dilution prior to administration.
Levofloxacin Injection is supplied in single-dose vials containing a concentrated levofloxacin solution with the equivalent of 500 mg (20 mL vial) and 750 mg (30 mL vial) of levofloxacin in Water for Injection, USP. The 20 mL and 30 mL vials each contain 25 mg of levofloxacin/mL. These Levofloxacin Injection single-dose vials must be further diluted with an appropriate solution prior to intravenous administration [
Compatible Intravenous Solutions: Any of the following intravenous solutions may be used to prepare a 5 mg/mL levofloxacin solution with the approximate pH values:
Intravenous Fluids | Final pH of Levofloxacin Solution |
| 0.9% Sodium Chloride Injection, USP | 4.71 |
| 5% Dextrose Injection, USP | 4.58 |
| 5% Dextrose/0.9% NaCl Injection | 4.62 |
| 5% Dextrose in Lactated Ringers | 4.92 |
| Plasma-Lyte® 56/5% Dextrose Injection | 5.03 |
| 5% Dextrose, 0.45% Sodium Chloride, and 0.15% Potassium Chloride Injection | 4.61 |
| Sodium Lactate Injection (M/6) | 5.54 |
Since no preservative or bacteriostatic agent is present in this product, aseptic technique must be used in preparation of the final intravenous solution. Since the vials are for single-dose only, any unused portion remaining in the vial should be discarded. When used to prepare two 250 mg doses from the 20 mL vial containing 500 mg of levofloxacin, the full content of the vial should be withdrawn at once using a single-entry procedure, and a second dose should be prepared and stored for subsequent use [see Stability of Levofloxacin Injection Following Dilution].
Prepare the desired dosage of levofloxacin according to the table below:
Desired Dosage Strength | From Appropriate Vial, Withdraw Volume | Volume of Diluent | Infusion Time |
| 250 mg | 10 mL (20 mL Vial) | 40 mL | 60 min |
| 500 mg | 20 mL (20 mL Vial) | 80 mL | 60 min |
| 750 mg | 30 mL (30 mL Vial) | 120 mL | 90 min |
For example, to prepare a 500 mg dose using the 20 mL vial (25 mg/mL), withdraw 20 mL and dilute with a compatible intravenous solution to a total volume of 100 mL.
This intravenous drug product should be inspected visually for particulate matter prior to administration. Samples containing visible particles should be discarded.
Stability of Levofloxacin Injection Following Dilution: Levofloxacin Injection, when diluted in a compatible intravenous fluid to a concentration of 5 mg/mL, is stable for 72 hours when stored at or below 25°C (77°F) and for 14 days when stored under refrigeration at 5°C (41°F) in plastic intravenous containers. Solutions that are diluted in a compatible intravenous solution and frozen in glass bottles or plastic intravenous containers are stable for 6 months when stored at -20°C (-4°F). Thaw frozen solutions at room temperature 25°C (77°F) or in a refrigerator 8°C (46°F). Do not force thaw by microwave irradiation or water bath immersion. Do not refreeze after initial thawing.
Levofloxacin injection, single-dose vials of concentrated solution for dilution for intravenous infusion, clear yellow to clear greenish-yellow in appearance.
- 20 mL vial of 25 mg/mL levofloxacin solution, equivalent to 500 mg of levofloxacin
- 30 mL vial of 25 mg/mL levofloxacin solution, equivalent to 750 mg of levofloxacin
•
Post-marketing reports of severe hepatotoxicity (including acute hepatitis and fatal events) have been received for patients treated with Levofloxacin. No evidence of serious drug-associated hepatotoxicity was detected in clinical trials of over 7,000 patients. Severe hepatotoxicity generally occurred within 14 days of initiation of therapy and most cases occurred within 6 days. Most cases of severe hepatotoxicity were not associated with hypersensitivity
Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone such as Levofloxacin. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involve the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing Levofloxacin to elderly patients especially those on corticosteroids. Patients should be informed of this potential side effect and advised to discontinue Levofloxacin and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur
In Phase 3 clinical trials, 1,945 Levofloxacin-treated patients (26%) were ≥ 65 years of age. Of these, 1,081 patients (14%) were between the ages of 65 and 74 and 864 patients (12%) were 75 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Severe, and sometimes fatal, cases of hepatotoxicity have been reported post-marketing in association with Levofloxacin. The majority of fatal hepatotoxicity reports occurred in patients 65 years of age or older and most were not associated with hypersensitivity. Levofloxacin should be discontinued immediately if the patient develops signs and symptoms of hepatitis
Epidemiologic studies report an increased rate of aortic aneurysm and dissection within two months following use of fluoroquinolones, particularly in elderly patients
Elderly patients may be more susceptible to drug-associated effects on the QT interval. Therefore, precaution should be taken when using Levofloxacin with concomitant drugs that can result in prolongation of the QT interval (e.g., Class IA or Class III antiarrhythmics) or in patients with risk factors for torsade de pointes (e.g., known QT prolongation, uncorrected hypokalemia)
The pharmacokinetic properties of levofloxacin in younger adults and elderly adults do not differ significantly when creatinine clearance is taken into consideration. However, since the drug is known to be substantially excreted by the kidney, the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Advise patients to stop taking levofloxacin if they experience an adverse reaction and to call their healthcare provider for advice on completing the full course of treatment with another antibacterial drug.
Inform patients of the following serious adverse reactions that have been associated with levofloxacin or other fluoroquinolone use:
- Disabling and Potentially Irreversible Serious Adverse Reactions That May Occur Together: Inform patients that disabling and potentially irreversible serious adverse reactions, including tendinitis and tendon rupture, peripheral neuropathies, and central nervous system effects, have been associated with use of levofloxacin and may occur together in the same patient. Inform patients to stop taking levofloxacin immediately if they experience an adverse reaction and to call their healthcare provider.
- Tendinitis and Tendon Rupture: Instruct patients to contact their healthcare provider if they experience pain, swelling, or inflammation of a tendon, or weakness or inability to use one of their joints; rest and refrain from exercise; and discontinue levofloxacin treatment. Symptoms may be irreversible. The risk of severe tendon disorder with fluoroquinolones is higher in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
- Peripheral Neuropathies: Inform patients that peripheral neuropathies have been associated with levofloxacin use, symptoms may occur soon after initiation of therapy and may be irreversible. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness and/or weakness develop, immediately discontinue levofloxacin and tell them to contact their physician.
- Central Nervous System Effects(for example, convulsions, dizziness, lightheadedness, increased intracranial pressure): Inform patients that convulsions have been reported in patients receiving fluoroquinolones, including levofloxacin. Instruct patients to notify their physician before taking this drug if they have a history of convulsions. Inform patients that they should know how they react to levofloxacin before they operate an automobile or machinery or engage in other activities requiring mental alertness and coordination. Instruct patients to notify their physician if persistent headache with or without blurred vision occurs.
- Exacerbation of Myasthenia Gravis: Instruct patients to inform their physician of any history of myasthenia gravis. Instruct patients to notify their physician if they experience any symptoms of muscle weakness, including respiratory difficulties.
- Hypersensitivity Reactions:Inform patients that levofloxacin can cause hypersensitivity reactions, even following a single dose, and to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat, difficulty in swallowing or breathing, any swelling suggesting angioedema (for example, swelling of the lips, tongue, face, tightness of the throat, hoarseness), or other symptoms of an allergic reaction.
- Hepatotoxicity: Inform patients that severe hepatotoxicity (including acute hepatitis and fatal events) has been reported in patients taking levofloxacin. Instruct patients to inform their physician if they experience any signs or symptoms of liver injury including: loss of appetite, nausea, vomiting, fever, weakness, tiredness, right upper quadrant tenderness, itching, yellowing of the skin and eyes, light colored bowel movements or dark colored urine.
- Aortic aneurysm and dissection:Inform patients to seek emergency medical care if they experience sudden chest, stomach, or back pain.
- Diarrhea: Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, instruct patients to contact their physician as soon as possible.
- Prolongation of the QT Interval: Instruct patients to inform their physician of any personal or family history of QT prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia; if they are taking any Class IA (quinidine, procainamide), or Class III (amiodarone, sotalol) antiarrhythmic agents. Instruct patients to notify their physician if they have any symptoms of prolongation of the QT interval, including prolonged heart palpitations or a loss of consciousness.
- Musculoskeletal Disorders in Pediatric Patients: Instruct parents to inform their child’s physician if the child has a history of joint-related problems before taking this drug. Inform parents of pediatric patients to notify their child’s physician of any joint-related problems that occur during or following levofloxacin therapy[see Warnings and Precautions (5.12) and Use in Specific Populations (8.4)].
- Photosensitivity/Phototoxicity: Inform patients that photosensitivity/phototoxicity has been reported in patients receiving fluoroquinolones. Inform patients to minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking fluoroquinolones. If patients need to be outdoors while using fluoroquinolones, instruct them to wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, instruct patients to contact their physician.
Antibacterial drugs including levofloxacin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When levofloxacin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by levofloxacin or other antibacterial drugs in the future.
Patients should drink fluids liberally while taking levofloxacin to avoid formation of a highly concentrated urine and crystal formation in the urine.
Patients should be informed that if they are diabetic and are being treated with insulin or an oral hypoglycemic agent and a hypoglycemic reaction occurs, they should discontinue levofloxacin and consult a physician.
Patients should be informed that concurrent administration of warfarin and levofloxacin has been associated with increases of the International Normalized Ratio (INR) or prothrombin time and clinical episodes of bleeding. Patients should notify their physician if they are taking warfarin, be monitored for evidence of bleeding, and also have their anticoagulation tests closely monitored while taking warfarin concomitantly.
Patients given levofloxacin for these conditions should be informed that efficacy studies could not be conducted in humans for ethical and feasibility reasons. Therefore, approval for these conditions was based on efficacy studies conducted in animals.
Manufactured by:
D.P.Pally, Hyderabad- 500 043
Telangana, India.
Revised: 09/2024
Some fluoroquinolones, including Levofloxacin, have been associated with prolongation of the QT interval on the electrocardiogram and infrequent cases of arrhythmia. Rare cases of torsade de pointes have been spontaneously reported during postmarketing surveillance in patients receiving fluoroquinolones, including Levofloxacin. Levofloxacin should be avoided in patients with known prolongation of the QT interval, patients with uncorrected hypokalemia, and patients receiving Class IA (quinidine, procainamide), or Class III (amiodarone, sotalol) antiarrhythmic agents. Elderly patients may be more susceptible to drug-associated effects on the QT interval
Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone such as Levofloxacin. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involve the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing Levofloxacin to elderly patients especially those on corticosteroids. Patients should be informed of this potential side effect and advised to discontinue Levofloxacin and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur
In Phase 3 clinical trials, 1,945 Levofloxacin-treated patients (26%) were ≥ 65 years of age. Of these, 1,081 patients (14%) were between the ages of 65 and 74 and 864 patients (12%) were 75 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Severe, and sometimes fatal, cases of hepatotoxicity have been reported post-marketing in association with Levofloxacin. The majority of fatal hepatotoxicity reports occurred in patients 65 years of age or older and most were not associated with hypersensitivity. Levofloxacin should be discontinued immediately if the patient develops signs and symptoms of hepatitis
Epidemiologic studies report an increased rate of aortic aneurysm and dissection within two months following use of fluoroquinolones, particularly in elderly patients
Elderly patients may be more susceptible to drug-associated effects on the QT interval. Therefore, precaution should be taken when using Levofloxacin with concomitant drugs that can result in prolongation of the QT interval (e.g., Class IA or Class III antiarrhythmics) or in patients with risk factors for torsade de pointes (e.g., known QT prolongation, uncorrected hypokalemia)
The pharmacokinetic properties of levofloxacin in younger adults and elderly adults do not differ significantly when creatinine clearance is taken into consideration. However, since the drug is known to be substantially excreted by the kidney, the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Advise patients to stop taking levofloxacin if they experience an adverse reaction and to call their healthcare provider for advice on completing the full course of treatment with another antibacterial drug.
Inform patients of the following serious adverse reactions that have been associated with levofloxacin or other fluoroquinolone use:
- Disabling and Potentially Irreversible Serious Adverse Reactions That May Occur Together: Inform patients that disabling and potentially irreversible serious adverse reactions, including tendinitis and tendon rupture, peripheral neuropathies, and central nervous system effects, have been associated with use of levofloxacin and may occur together in the same patient. Inform patients to stop taking levofloxacin immediately if they experience an adverse reaction and to call their healthcare provider.
- Tendinitis and Tendon Rupture: Instruct patients to contact their healthcare provider if they experience pain, swelling, or inflammation of a tendon, or weakness or inability to use one of their joints; rest and refrain from exercise; and discontinue levofloxacin treatment. Symptoms may be irreversible. The risk of severe tendon disorder with fluoroquinolones is higher in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
- Peripheral Neuropathies: Inform patients that peripheral neuropathies have been associated with levofloxacin use, symptoms may occur soon after initiation of therapy and may be irreversible. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness and/or weakness develop, immediately discontinue levofloxacin and tell them to contact their physician.
- Central Nervous System Effects(for example, convulsions, dizziness, lightheadedness, increased intracranial pressure): Inform patients that convulsions have been reported in patients receiving fluoroquinolones, including levofloxacin. Instruct patients to notify their physician before taking this drug if they have a history of convulsions. Inform patients that they should know how they react to levofloxacin before they operate an automobile or machinery or engage in other activities requiring mental alertness and coordination. Instruct patients to notify their physician if persistent headache with or without blurred vision occurs.
- Exacerbation of Myasthenia Gravis: Instruct patients to inform their physician of any history of myasthenia gravis. Instruct patients to notify their physician if they experience any symptoms of muscle weakness, including respiratory difficulties.
- Hypersensitivity Reactions:Inform patients that levofloxacin can cause hypersensitivity reactions, even following a single dose, and to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat, difficulty in swallowing or breathing, any swelling suggesting angioedema (for example, swelling of the lips, tongue, face, tightness of the throat, hoarseness), or other symptoms of an allergic reaction.
- Hepatotoxicity: Inform patients that severe hepatotoxicity (including acute hepatitis and fatal events) has been reported in patients taking levofloxacin. Instruct patients to inform their physician if they experience any signs or symptoms of liver injury including: loss of appetite, nausea, vomiting, fever, weakness, tiredness, right upper quadrant tenderness, itching, yellowing of the skin and eyes, light colored bowel movements or dark colored urine.
- Aortic aneurysm and dissection:Inform patients to seek emergency medical care if they experience sudden chest, stomach, or back pain.
- Diarrhea: Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, instruct patients to contact their physician as soon as possible.
- Prolongation of the QT Interval: Instruct patients to inform their physician of any personal or family history of QT prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia; if they are taking any Class IA (quinidine, procainamide), or Class III (amiodarone, sotalol) antiarrhythmic agents. Instruct patients to notify their physician if they have any symptoms of prolongation of the QT interval, including prolonged heart palpitations or a loss of consciousness.
- Musculoskeletal Disorders in Pediatric Patients: Instruct parents to inform their child’s physician if the child has a history of joint-related problems before taking this drug. Inform parents of pediatric patients to notify their child’s physician of any joint-related problems that occur during or following levofloxacin therapy[see Warnings and Precautions (5.12) and Use in Specific Populations (8.4)].
- Photosensitivity/Phototoxicity: Inform patients that photosensitivity/phototoxicity has been reported in patients receiving fluoroquinolones. Inform patients to minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking fluoroquinolones. If patients need to be outdoors while using fluoroquinolones, instruct them to wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, instruct patients to contact their physician.
Antibacterial drugs including levofloxacin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When levofloxacin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by levofloxacin or other antibacterial drugs in the future.
Patients should drink fluids liberally while taking levofloxacin to avoid formation of a highly concentrated urine and crystal formation in the urine.
Patients should be informed that if they are diabetic and are being treated with insulin or an oral hypoglycemic agent and a hypoglycemic reaction occurs, they should discontinue levofloxacin and consult a physician.
Patients should be informed that concurrent administration of warfarin and levofloxacin has been associated with increases of the International Normalized Ratio (INR) or prothrombin time and clinical episodes of bleeding. Patients should notify their physician if they are taking warfarin, be monitored for evidence of bleeding, and also have their anticoagulation tests closely monitored while taking warfarin concomitantly.
Patients given levofloxacin for these conditions should be informed that efficacy studies could not be conducted in humans for ethical and feasibility reasons. Therefore, approval for these conditions was based on efficacy studies conducted in animals.
Manufactured by:
D.P.Pally, Hyderabad- 500 043
Telangana, India.
Revised: 09/2024
•
Levofloxacin is indicated in pediatric patients (6 months of age and older) only for the prevention of inhalational anthrax (post-exposure) and for plague
In immature rats and dogs, intravenous administration of levofloxacin resulted in increased osteochondrosis. Histopathological examination of the weight-bearing joints of immature dogs dosed with levofloxacin revealed persistent lesions of the cartilage. Other fluoroquinolones also produce similar erosions in the weight-bearing joints and other signs of arthropathy in immature animals of various species
Quinolones, including levofloxacin, cause arthropathy and osteochondrosis in juvenile animals of several species
The pharmacokinetics of levofloxacin following a single intravenous dose were investigated in pediatric patients ranging in age from six months to 16 years. Pediatric patients cleared levofloxacin faster than adult patients resulting in lower plasma exposures than adults for a given mg/kg dose
Levofloxacin is indicated in pediatric patients 6 months of age and older, for inhalational anthrax (post-exposure). The risk-benefit assessment indicates that administration of levofloxacin to pediatric patients is appropriate. The safety of levofloxacin in pediatric patients treated for more than 14 days has not been studied
Levofloxacin is indicated in pediatric patients, 6 months of age and older, for treatment of plague, including pneumonic and septicemic plague due to
Safety and effectiveness in pediatric patients below the age of six months have not been established.
In clinical trials, 1,534 children (6 months to 16 years of age) were treated with intravenous Levofloxacin. Children 6 months to 5 years of age received Levofloxacin 10 mg/kg twice a day and children greater than 5 years of age received 10 mg/kg once a day (maximum 500 mg per day) for approximately 10 days.
A subset of children in the clinical trials (1,340 Levofloxacin-treated and 893 non-fluoroquinolone-treated) enrolled in a prospective, long-term surveillance study to assess the incidence of protocol-defined musculoskeletal disorders (arthralgia, arthritis, tendinopathy, gait abnormality) during 60 days and 1 year following the first dose of the study drug. Children treated with Levofloxacin had a significantly higher incidence of musculoskeletal disorders when compared to the non-fluoroquinolone-treated children as illustrated in Table 9.
Follow-up Period | Levofloxacin N = 1,340 | Non-Fluoroquinolone* N = 893 | p-value† |
| 60 days | 28 (2.1%) | 8 (0.9%) | p = 0.038 |
| 1 year‡ | 46 (3.4%) | 16 (1.8%) | p = 0.025 |
* Non-Fluoroquinolone: ceftriaxone, amoxicillin/clavulanate, clarithromycin
†2-sided Fisher’s Exact Test
‡There were 1,199 Levofloxacin-treated and 804 non-fluoroquinolone-treated children who had a one-year evaluation visit. However, the incidence of musculoskeletal disorders was calculated using all reported events during the specified period for all children enrolled regardless of whether they completed the 1-year evaluation visit.
Arthralgia was the most frequently occurring musculoskeletal disorder in both treatment groups. Most of the musculoskeletal disorders in both groups involved multiple weight-bearing joints. Disorders were moderate in 8/46 (17%) children and mild in 35/46 (76%) Levofloxacin treated children and most were treated with analgesics. The median time to resolution was 7 days for Levofloxacin-treated children and 9 for non-fluoroquinolone-treated children (approximately 80% resolved within 2 months in both groups). No child had a severe or serious disorder and all musculoskeletal disorders resolved without sequelae.
Vomiting and diarrhea were the most frequently reported adverse events, occurring in similar frequency in the Levofloxacin-treated and non-fluoroquinolone-treated children.
In addition to the events reported in pediatric patients in clinical trials, events reported in adults during clinical trials or post-marketing experience [see Adverse Reactions (6)] may also be expected to occur in pediatric patients.
Levofloxacin and other quinolones have been shown to cause arthropathy in immature animals of most species tested
When tested in a mouse ear swelling bioassay, levofloxacin exhibited phototoxicity similar in magnitude to ofloxacin, but less phototoxicity than other quinolones.
While crystalluria has been observed in some intravenous rat studies, urinary crystals are not formed in the bladder, being present only after micturition and are not associated with nephrotoxicity.
In mice, the CNS stimulatory effect of quinolones is enhanced by concomitant administration of non-steroidal anti-inflammatory drugs.
In dogs, levofloxacin administered at 6 mg/kg or higher by rapid intravenous injection produced hypotensive effects. These effects were considered to be related to histamine release.
Levofloxacin is indicated for inhalational anthrax (post-exposure) to reduce the incidence or progression of disease following exposure to aerosolized
The dosage in pediatric patients ≥ 6 months of age is described below in Table 2.
Type of Infection* | Dose | Freq. Once every | Duration† |
| Inhalational Anthrax (post-exposure)‡,§ | |||
| Pediatric patients > 50 kg | 500 mg | 24 hr | 60 days§ |
| Pediatric patients < 50 kg and ≥ 6 months of age | 8 mg/kg (not to exceed 250 mg per dose) | 12 hr | 60 days§ |
| Plague¶ | |||
| Pediatric patients > 50 kg | 500 mg | 24 hr | 10 to 14 days |
| Pediatric patients < 50 kg and ≥ 6 months of age | 8 mg/kg (not to exceed 250 mg per dose) | 12 hr | 10 to 14 days |
* Due to
†Sequential therapy (intravenous to oral) may be instituted at the discretion of the physician.
‡ Drug administration should begin as soon as possible after suspected or confirmed exposure to aerosolized
§The safety of Levofloxacin in pediatric patients for durations of therapy beyond 14 days has not been studied. An increased incidence of musculoskeletal adverse events compared to controls has been observed in pediatric patients
¶Drug administration should begin as soon as possible after suspected or confirmed exposure to
Quinolones, including levofloxacin, cause arthropathy and osteochondrosis in juvenile animals of several species
The pharmacokinetics of levofloxacin following a single intravenous dose were investigated in pediatric patients ranging in age from six months to 16 years. Pediatric patients cleared levofloxacin faster than adult patients resulting in lower plasma exposures than adults for a given mg/kg dose
Levofloxacin is indicated in pediatric patients 6 months of age and older, for inhalational anthrax (post-exposure). The risk-benefit assessment indicates that administration of levofloxacin to pediatric patients is appropriate. The safety of levofloxacin in pediatric patients treated for more than 14 days has not been studied
Levofloxacin is indicated in pediatric patients, 6 months of age and older, for treatment of plague, including pneumonic and septicemic plague due to
Safety and effectiveness in pediatric patients below the age of six months have not been established.
In clinical trials, 1,534 children (6 months to 16 years of age) were treated with intravenous Levofloxacin. Children 6 months to 5 years of age received Levofloxacin 10 mg/kg twice a day and children greater than 5 years of age received 10 mg/kg once a day (maximum 500 mg per day) for approximately 10 days.
A subset of children in the clinical trials (1,340 Levofloxacin-treated and 893 non-fluoroquinolone-treated) enrolled in a prospective, long-term surveillance study to assess the incidence of protocol-defined musculoskeletal disorders (arthralgia, arthritis, tendinopathy, gait abnormality) during 60 days and 1 year following the first dose of the study drug. Children treated with Levofloxacin had a significantly higher incidence of musculoskeletal disorders when compared to the non-fluoroquinolone-treated children as illustrated in Table 9.
Follow-up Period | Levofloxacin N = 1,340 | Non-Fluoroquinolone* N = 893 | p-value† |
| 60 days | 28 (2.1%) | 8 (0.9%) | p = 0.038 |
| 1 year‡ | 46 (3.4%) | 16 (1.8%) | p = 0.025 |
* Non-Fluoroquinolone: ceftriaxone, amoxicillin/clavulanate, clarithromycin
†2-sided Fisher’s Exact Test
‡There were 1,199 Levofloxacin-treated and 804 non-fluoroquinolone-treated children who had a one-year evaluation visit. However, the incidence of musculoskeletal disorders was calculated using all reported events during the specified period for all children enrolled regardless of whether they completed the 1-year evaluation visit.
Arthralgia was the most frequently occurring musculoskeletal disorder in both treatment groups. Most of the musculoskeletal disorders in both groups involved multiple weight-bearing joints. Disorders were moderate in 8/46 (17%) children and mild in 35/46 (76%) Levofloxacin treated children and most were treated with analgesics. The median time to resolution was 7 days for Levofloxacin-treated children and 9 for non-fluoroquinolone-treated children (approximately 80% resolved within 2 months in both groups). No child had a severe or serious disorder and all musculoskeletal disorders resolved without sequelae.
Vomiting and diarrhea were the most frequently reported adverse events, occurring in similar frequency in the Levofloxacin-treated and non-fluoroquinolone-treated children.
In addition to the events reported in pediatric patients in clinical trials, events reported in adults during clinical trials or post-marketing experience [see Adverse Reactions (6)] may also be expected to occur in pediatric patients.
The effectiveness of Levofloxacin for this indication is based on plasma concentrations achieved in humans, a surrogate endpoint reasonably likely to predict clinical benefit. Levofloxacin has not been tested in humans for the post-exposure prevention of inhalation anthrax. The mean plasma concentrations of Levofloxacin associated with a statistically significant improvement in survival over placebo in the rhesus monkey model of inhalational anthrax are reached or exceeded in adult and pediatric patients receiving the recommended oral and intravenous dosage regimens
Levofloxacin pharmacokinetics have been evaluated in adult and pediatric patients. The mean (± SD) steady state peak plasma concentration in human adults receiving 500 mg orally or intravenously once daily is 5.7 ± 1.4 and 6.4 ± 0.8 mcg/mL, respectively; and the corresponding total plasma exposure (AUC0-24) is 47.5 ± 6.7 and 54.6 ± 11.1 mcg.h/mL, respectively. The predicted steady-state pharmacokinetic parameters in pediatric patients ranging in age from 6 months to 17 years receiving 8 mg/kg orally every 12 hours (not to exceed 250 mg per dose) were calculated to be comparable to those observed in adults receiving 500 mg orally once daily
In adults, the safety of Levofloxacin for treatment durations of up to 28 days is well characterized. However, information pertaining to extended use at 500 mg daily up to 60 days is limited. Prolonged Levofloxacin therapy in adults should only be used when the benefit outweighs the risk.
In pediatric patients, the safety of levofloxacin for treatment durations of more than 14 days has not been studied. An increased incidence of musculoskeletal adverse events (arthralgia, arthritis, tendinopathy, gait abnormality) compared to controls has been observed in clinical studies with treatment duration of up to 14 days. Long-term safety data, including effects on cartilage, following the administration of levofloxacin to pediatric patients is limited
A placebo-controlled animal study in rhesus monkeys exposed to an inhaled mean dose of 49 LD50(~2.7 × 106) spores (range 17 – 118 LD50) of
Levofloxacin is indicated for treatment of plague, including pneumonic and septicemic plague, due to
The dosage in pediatric patients ≥ 6 months of age is described below in Table 2.
Type of Infection* | Dose | Freq. Once every | Duration† |
| Inhalational Anthrax (post-exposure)‡,§ | |||
| Pediatric patients > 50 kg | 500 mg | 24 hr | 60 days§ |
| Pediatric patients < 50 kg and ≥ 6 months of age | 8 mg/kg (not to exceed 250 mg per dose) | 12 hr | 60 days§ |
| Plague¶ | |||
| Pediatric patients > 50 kg | 500 mg | 24 hr | 10 to 14 days |
| Pediatric patients < 50 kg and ≥ 6 months of age | 8 mg/kg (not to exceed 250 mg per dose) | 12 hr | 10 to 14 days |
* Due to
†Sequential therapy (intravenous to oral) may be instituted at the discretion of the physician.
‡ Drug administration should begin as soon as possible after suspected or confirmed exposure to aerosolized
§The safety of Levofloxacin in pediatric patients for durations of therapy beyond 14 days has not been studied. An increased incidence of musculoskeletal adverse events compared to controls has been observed in pediatric patients
¶Drug administration should begin as soon as possible after suspected or confirmed exposure to
Quinolones, including levofloxacin, cause arthropathy and osteochondrosis in juvenile animals of several species
The pharmacokinetics of levofloxacin following a single intravenous dose were investigated in pediatric patients ranging in age from six months to 16 years. Pediatric patients cleared levofloxacin faster than adult patients resulting in lower plasma exposures than adults for a given mg/kg dose
Levofloxacin is indicated in pediatric patients 6 months of age and older, for inhalational anthrax (post-exposure). The risk-benefit assessment indicates that administration of levofloxacin to pediatric patients is appropriate. The safety of levofloxacin in pediatric patients treated for more than 14 days has not been studied
Levofloxacin is indicated in pediatric patients, 6 months of age and older, for treatment of plague, including pneumonic and septicemic plague due to
Safety and effectiveness in pediatric patients below the age of six months have not been established.
In clinical trials, 1,534 children (6 months to 16 years of age) were treated with intravenous Levofloxacin. Children 6 months to 5 years of age received Levofloxacin 10 mg/kg twice a day and children greater than 5 years of age received 10 mg/kg once a day (maximum 500 mg per day) for approximately 10 days.
A subset of children in the clinical trials (1,340 Levofloxacin-treated and 893 non-fluoroquinolone-treated) enrolled in a prospective, long-term surveillance study to assess the incidence of protocol-defined musculoskeletal disorders (arthralgia, arthritis, tendinopathy, gait abnormality) during 60 days and 1 year following the first dose of the study drug. Children treated with Levofloxacin had a significantly higher incidence of musculoskeletal disorders when compared to the non-fluoroquinolone-treated children as illustrated in Table 9.
Follow-up Period | Levofloxacin N = 1,340 | Non-Fluoroquinolone* N = 893 | p-value† |
| 60 days | 28 (2.1%) | 8 (0.9%) | p = 0.038 |
| 1 year‡ | 46 (3.4%) | 16 (1.8%) | p = 0.025 |
* Non-Fluoroquinolone: ceftriaxone, amoxicillin/clavulanate, clarithromycin
†2-sided Fisher’s Exact Test
‡There were 1,199 Levofloxacin-treated and 804 non-fluoroquinolone-treated children who had a one-year evaluation visit. However, the incidence of musculoskeletal disorders was calculated using all reported events during the specified period for all children enrolled regardless of whether they completed the 1-year evaluation visit.
Arthralgia was the most frequently occurring musculoskeletal disorder in both treatment groups. Most of the musculoskeletal disorders in both groups involved multiple weight-bearing joints. Disorders were moderate in 8/46 (17%) children and mild in 35/46 (76%) Levofloxacin treated children and most were treated with analgesics. The median time to resolution was 7 days for Levofloxacin-treated children and 9 for non-fluoroquinolone-treated children (approximately 80% resolved within 2 months in both groups). No child had a severe or serious disorder and all musculoskeletal disorders resolved without sequelae.
Vomiting and diarrhea were the most frequently reported adverse events, occurring in similar frequency in the Levofloxacin-treated and non-fluoroquinolone-treated children.
In addition to the events reported in pediatric patients in clinical trials, events reported in adults during clinical trials or post-marketing experience [see Adverse Reactions (6)] may also be expected to occur in pediatric patients.
Efficacy studies of Levofloxacin could not be conducted in humans with pneumonic plague for ethical and feasibility reasons. Therefore, approval of this indication was based on an efficacy study conducted in animals. The mean plasma concentrations of Levofloxacin associated with a statistically significant improvement in survival over placebo in an African green monkey model of pneumonic plague are reached or exceeded in adult and pediatric patients receiving the recommended oral and intravenous dosage regimens
Levofloxacin pharmacokinetics have been evaluated in adult and pediatric patients. The mean (± SD) steady state peak plasma concentration in human adults receiving 500 mg orally or intravenously once daily is 5.7 ± 1.4 and 6.4 ± 0.8 mcg/mL, respectively; and the corresponding total plasma exposure (AUC0-24) is 47.5 ± 6.7 and 54.6 ± 11.1 mcg.h/mL, respectively.
The predicted steady-state pharmacokinetic parameters in pediatric patients ranging in age from 6 months to 17 years receiving 8 mg/kg orally every 12 hours (not to exceed 250 mg per dose) were calculated to be comparable to those observed in adults receiving 500 mg orally once daily
A placebo-controlled animal study in African green monkeys exposed to an inhaled mean dose of 65 LD50 (range 3 to 145 LD50) of