Levonorgestrel And Ethinyl Estradiol Prescribing Information
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs, including levonorgestrel and ethinyl estradiol tablets, are contraindicated in women who are over 35 years of age and smoke [see
Levonorgestrel and ethinyl estradiol tablets are contraindicated in females who are known to have the following conditions:
• A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:o Smoke, if over age 35 [see BOXED WARNINGand WARNINGS (1)].o Have current or history of deep vein thrombosis or pulmonary embolism [see WARNINGS (1)].o Have cerebrovascular disease [see WARNINGS (1)].o Have coronary artery disease [see WARNINGS (1)].o Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see WARNINGS (1)].o Have inherited or acquired hypercoagulopathies [see WARNINGS (1)].o Have uncontrolled hypertension or hypertension with vascular disease [see WARNINGS (3)].o Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or vascular disease or other end-organ damage, or diabetes mellitus of >20 years duration [see WARNINGS (7)].o Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches [see WARNINGS (8)].
• Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive.• Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis [see WARNINGS (2)].• Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations [see WARNINGS (5)].• Undiagnosed abnormal uterine bleeding [see WARNINGS (9)].
• Stop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.• Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.• Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least four weeks before and through two weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.• Start levonorgestrel and ethinyl estradiol tablets no earlier than four weeks after delivery in females who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.• Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases (see CONTRAINDICATIONS).
COCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke (see CONTRAINDICATIONS). Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
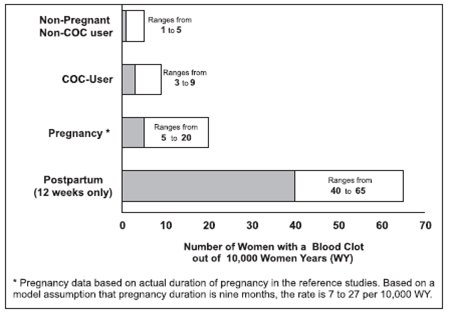
Use of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs (see CONTRAINDICATIONS). While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1 Likelihood of Developing a VTE

Levonorgestrel and ethinyl estradiol tablets, 0.15 mg/30 mcg are indicated for use by females of reproductive potential to prevent pregnancy.
Levonorgestrel and ethinyl estradiol tablets, 0.15 mg/30 mcg are dispensed in a compact dispenser containing 28 tablets (see
Levonorgestrel and Ethinyl Estradiol Tablets USP, 0.15 mg/30 mcg are available in a carton of 3 pouches and a carton of 6 pouches, each pouch contains 28 tablets:
21 Active Tablets: | White to off-white, round, unscored tablets debossed with 209 on one side and plain on the other side. |
7 Inert Tablets: | Green, round, unscored tablets debossed with 274 on one side and plain on the other side. |
NDC 0378-6550-53, cartons of 3 pouches, each pouch containing 28 tablets | |
NDC 0378-6550-56, cartons of 6 pouches, each pouch containing 28 tablets |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
• After a first-trimester abortion or miscarriage, levonorgestrel and ethinyl estradiol tablets may be started immediately. An additional method of contraception is not needed if levonorgestrel and ethinyl estradiol tablets are started immediately.• If levonorgestrel and ethinyl estradiol tablets are not started within 5 days after termination of the pregnancy, the patient should use additional non-hormonal contraception (such as condoms or spermicide) for the first seven days of her first cycle of levonorgestrel and ethinyl estradiol tablets.
• Do not start until 4 weeks after a second-trimester abortion or miscarriage, due to the increased risk of thromboembolic disease. Start levonorgestrel and ethinyl estradiol tablets following the instructions infor Day 1 or Sunday start. Use additional non-hormonal contraception (such as condoms or spermicide) for the first seven days of the patient’s first cycle of levonorgestrel and ethinyl estradiol tablets (seeTable 3: Instructions for Administration of Levonorgestrel and Ethinyl Estradiol Tablets - Starting levonorgestrel and ethinyl estradiol tabletsin females with no current use of hormonal contraception
- Day 1 start
• Take first tablet without regard to meals on the first day of menses• Take subsequent tablets once daily at the same time each day• Begin each subsequent pack on the same day of the week as the first cycle pack (i.e., on the day after taking the last tablet)
- Sunday start
• Take first tablet without regard to meals on the first Sunday after the onset of menstrual period• Take subsequent tablets once daily at the same time each day• Use additional nonhormonal contraception for the first seven days of product use• Begin each subsequent pack on the same day of the week as the first cycle pack (i.e., on the day after taking the last tablet)
- Switching from another contraceptive method
• A COC
• Start levonorgestrel and ethinyl estradiol tablets:• On the day when the new pack of the previous COC would have been started
• Transdermal patch
• On the day when next application would have been scheduled
• Vaginal ring
• On the day when next insertion would have been scheduled
• Injection
• On the day when next injection would have been scheduled
• Intrauterine contraceptive
• On the day of removal
• Implant
• On the day of removal
,CONTRAINDICATIONSLevonorgestrel and ethinyl estradiol tablets are contraindicated in females who are known to have the following conditions:
• A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:o Smoke, if over age 35 [see BOXED WARNINGand WARNINGS (1)].o Have current or history of deep vein thrombosis or pulmonary embolism [see WARNINGS (1)].o Have cerebrovascular disease [see WARNINGS (1)].o Have coronary artery disease [see WARNINGS (1)].o Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see WARNINGS (1)].o Have inherited or acquired hypercoagulopathies [see WARNINGS (1)].o Have uncontrolled hypertension or hypertension with vascular disease [see WARNINGS (3)].o Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or vascular disease or other end-organ damage, or diabetes mellitus of >20 years duration [see WARNINGS (7)].o Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches [see WARNINGS (8)].
• Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive.• Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis [see WARNINGS (2)].• Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations [see WARNINGS (5)].• Undiagnosed abnormal uterine bleeding [see WARNINGS (9)].
,1. Thromboembolic Disorders and Other Vascular Conditions• Stop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.• Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.• Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least four weeks before and through two weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.• Start levonorgestrel and ethinyl estradiol tablets no earlier than four weeks after delivery in females who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.• Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases (see CONTRAINDICATIONS).
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke (see CONTRAINDICATIONS). Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs (see CONTRAINDICATIONS). While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1 Likelihood of Developing a VTE


Fig 01 and10. PATIENT COUNSELING INFORMATION• Counsel patients that cigarette smoking increases the risk of serious cardiovascular events from COC use, and that women who are over 35 years old and smoke should not use COCs (see BOXED WARNINGand CONTRAINDICATIONS).• Counsel patients that this product does not protect against HIV-infection (AIDS) and other sexually transmitted infections.• Counsel patients to take one tablet daily by mouth at the same time every day. Instruct patients what to do in the event pills are missed (see DOSAGE AND ADMINISTRATION).• Counsel patients to use a back-up or alternative method of contraception when enzyme inducers are used with COCs [see PRECAUTIONS (4.1)].• Counsel patients who are breastfeeding or who desire to breastfeed that COCs may reduce breast milk production. This is less likely to occur if breastfeeding is well established [see PRECAUTIONS (7)].• Counsel any patient who starts levonorgestrel and ethinyl estradiol tablets postpartum, and who has not yet had a period, to use an additional method of contraception until she has taken a white to off-white tablet for 7 consecutive days (see DOSAGE AND ADMINISTRATION).• Counsel patients that amenorrhea may occur. Pregnancy should be considered in the event of amenorrhea, and should be ruled out if amenorrhea is associated with symptoms of pregnancy, such as morning sickness or unusual breast tenderness [see WARNINGS (9)].• Depression may occur. Women should contact their healthcare provider if depression occurs, including shortly after initiating the treatment [see WARNINGS (10)].
).Patient Information
• Do not start until 4 weeks after delivery, due to the increased risk of thromboembolic disease. Start contraceptive therapy with levonorgestrel and ethinyl estradiol tablets following the instructions in Table 3 for women not currently using hormonal contraception.• Levonorgestrel and ethinyl estradiol tablets are not recommended for use in lactating women (seeand7. LactationRisk SummaryContraceptive hormones and/or metabolites are present in human milk. COCs can reduce milk production in breast-feeding females. This reduction can occur at any time but is less likely to occur once breast-feeding is well-established. When possible, advise the nursing female to use other methods of contraception until she discontinues breast-feeding. (see DOSAGE AND ADMINISTRATION). The developmental and health benefits of breast-feeding should be considered along with the mother’s clinical need for levonorgestrel and ethinyl estradiol tablets and any potential adverse effects on the breast-fed child from levonorgestrel and ethinyl estradiol tablets or from the underlying maternal condition.
).Patient Information• If the woman has not yet had a period postpartum, consider the possibility of ovulation and conception occurring prior to use of levonorgestrel and ethinyl estradiol tablets (see,CONTRAINDICATIONSLevonorgestrel and ethinyl estradiol tablets are contraindicated in females who are known to have the following conditions:
• A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:o Smoke, if over age 35 [see BOXED WARNINGand WARNINGS (1)].o Have current or history of deep vein thrombosis or pulmonary embolism [see WARNINGS (1)].o Have cerebrovascular disease [see WARNINGS (1)].o Have coronary artery disease [see WARNINGS (1)].o Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see WARNINGS (1)].o Have inherited or acquired hypercoagulopathies [see WARNINGS (1)].o Have uncontrolled hypertension or hypertension with vascular disease [see WARNINGS (3)].o Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or vascular disease or other end-organ damage, or diabetes mellitus of >20 years duration [see WARNINGS (7)].o Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches [see WARNINGS (8)].
• Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive.• Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis [see WARNINGS (2)].• Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations [see WARNINGS (5)].• Undiagnosed abnormal uterine bleeding [see WARNINGS (9)].
,9. Bleeding Irregularities and AmenorrheaUnscheduled Bleeding and SpottingFemales using levonorgestrel and ethinyl estradiol tablets may experience unscheduled (breakthrough or intracyclic) bleeding and spotting, especially during the first three months of use. Bleeding irregularities may resolve over time or by changing to a different contraceptive product. If bleeding persists or occurs after previously regular cycles, evaluate for causes such as pregnancy or malignancy.
In two clinical trials of levonorgestrel and ethinyl estradiol tablets (1084 subjects reporting for a total of 8186 treatment cycles and 238 subjects reporting for a total of 1102 treatment cycles), breakthrough bleeding occurred in 6.9% and 8.1% of reported cycles, and spotting occurred in 8.6% and 7.9% of reported cycles over the total study duration, respectively. In the two trials, intermenstrual bleeding (i.e., breakthrough bleeding and/or spotting) occurred in 13.1% and 12.9% of reported cycles over the total study duration, respectively. In one trial, 33 subjects out of 1084 (3.0%) discontinued due to bleeding irregularities (i.e., breakthrough bleeding and spotting); in the other trial, 6 subjects out of 238 (2.5%) discontinued due to bleeding irregularities.
Amenorrhea and OligomenorrheaFemales who use levonorgestrel and ethinyl estradiol tablets may experience absence of scheduled (withdrawal) bleeding, even if they are not pregnant. In two clinical trials of levonorgestrel and ethinyl estradiol tablets, one including 8186 reported treatment cycles, and the other including 1102 reported treatment cycles, amenorrhea occurred in 1.5% of treatment cycles in each trial.
If scheduled bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or two active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and perform appropriate diagnostic measures. If the patient has adhered to the prescribed dosing schedule and misses two consecutive periods, rule out pregnancy.
After discontinuation of a COC, amenorrhea or oligomenorrhea may occur, especially if these conditions were pre-existent.
and6. PregnancyRisk SummaryDiscontinue levonorgestrel and ethinyl estradiol tablets if pregnancy occurs because there is no reason to use COCs in pregnancy. Epidemiologic studies and meta-analyses have not found an increased risk of genital or nongenital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to COCs before conception or during early pregnancy. Animal studies to evaluate embryo/fetal toxicity were not conducted.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4 percent and 15 to 20 percent, respectively.
).Patient Information
Levonorgestrel and ethinyl estradiol tablets are contraindicated in females who are known to have the following conditions:
• A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:o Smoke, if over age 35 [seeandWARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTSCigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs, including levonorgestrel and ethinyl estradiol tablets, are contraindicated in women who are over 35 years of age and smoke [see CONTRAINDICATIONSand WARNINGS (1)].
].1. Thromboembolic Disorders and Other Vascular Conditions• Stop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.• Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.• Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least four weeks before and through two weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.• Start levonorgestrel and ethinyl estradiol tablets no earlier than four weeks after delivery in females who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.• Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases (see CONTRAINDICATIONS).
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke (see CONTRAINDICATIONS). Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs (see CONTRAINDICATIONS). While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1 Likelihood of Developing a VTE


Fig 01 o Have current or history of deep vein thrombosis or pulmonary embolism [see].1. Thromboembolic Disorders and Other Vascular Conditions• Stop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.• Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.• Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least four weeks before and through two weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.• Start levonorgestrel and ethinyl estradiol tablets no earlier than four weeks after delivery in females who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.• Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases (see CONTRAINDICATIONS).
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke (see CONTRAINDICATIONS). Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs (see CONTRAINDICATIONS). While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1 Likelihood of Developing a VTE


Fig 01 o Have cerebrovascular disease [see].1. Thromboembolic Disorders and Other Vascular Conditions• Stop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.• Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.• Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least four weeks before and through two weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.• Start levonorgestrel and ethinyl estradiol tablets no earlier than four weeks after delivery in females who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.• Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases (see CONTRAINDICATIONS).
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke (see CONTRAINDICATIONS). Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs (see CONTRAINDICATIONS). While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1 Likelihood of Developing a VTE


Fig 01 o Have coronary artery disease [see].1. Thromboembolic Disorders and Other Vascular Conditions• Stop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.• Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.• Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least four weeks before and through two weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.• Start levonorgestrel and ethinyl estradiol tablets no earlier than four weeks after delivery in females who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.• Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases (see CONTRAINDICATIONS).
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke (see CONTRAINDICATIONS). Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs (see CONTRAINDICATIONS). While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1 Likelihood of Developing a VTE


Fig 01 o Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see].1. Thromboembolic Disorders and Other Vascular Conditions• Stop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.• Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.• Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least four weeks before and through two weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.• Start levonorgestrel and ethinyl estradiol tablets no earlier than four weeks after delivery in females who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.• Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases (see CONTRAINDICATIONS).
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke (see CONTRAINDICATIONS). Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs (see CONTRAINDICATIONS). While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1 Likelihood of Developing a VTE


Fig 01 o Have inherited or acquired hypercoagulopathies [see].1. Thromboembolic Disorders and Other Vascular Conditions• Stop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.• Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.• Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least four weeks before and through two weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.• Start levonorgestrel and ethinyl estradiol tablets no earlier than four weeks after delivery in females who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.• Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases (see CONTRAINDICATIONS).
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke (see CONTRAINDICATIONS). Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs (see CONTRAINDICATIONS). While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1 Likelihood of Developing a VTE


Fig 01 o Have uncontrolled hypertension or hypertension with vascular disease [see].3. HypertensionLevonorgestrel and ethinyl estradiol tablets are contraindicated in females with uncontrolled hypertension or hypertension with vascular disease (see CONTRAINDICATIONS). For all females, including those with well-controlled hypertension, monitor blood pressure at routine visits and stop levonorgestrel and ethinyl estradiol tablets if blood pressure rises significantly.
An increase in blood pressure has been reported in females using COCs, and this increase is more likely in older women with extended duration of use. The effect of COCs on blood pressure may vary according to the progestin in the COC.
o Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or vascular disease or other end-organ damage, or diabetes mellitus of >20 years duration [see].7. Adverse Carbohydrate and Lipid Metabolic EffectsHyperglycemiaLevonorgestrel and ethinyl estradiol tablets are contraindicated in diabetic women over age 35, or females who have diabetes with hypertension, nephropathy, retinopathy, neuropathy, other vascular disease, or females with diabetes of > 20 years duration (see CONTRAINDICATIONS). Levonorgestrel and ethinyl estradiol tablets may decrease glucose tolerance. Carefully monitor prediabetic and diabetic females who are using levonorgestrel and ethinyl estradiol tablets.
DyslipidemiaConsider alternative contraception for females with uncontrolled dyslipidemia. Levonorgestrel and ethinyl estradiol tablets may cause adverse lipid changes.
Females with hypertriglyceridemia, or a family history thereof, may have an increase in serum triglyceride concentrations when using levonorgestrel and ethinyl estradiol tablets which may increase the risk of pancreatitis.
o Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches [see].8. HeadacheLevonorgestrel and ethinyl estradiol tablets are contraindicated in females who have headaches with focal neurological symptoms or have migraine headaches with aura, and in women over age 35 years who have migraine headaches with or without aura (see CONTRAINDICATIONS).
If a woman using levonorgestrel and ethinyl estradiol tablets develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue levonorgestrel and ethinyl estradiol tablets if indicated. Consider discontinuation of levonorgestrel and ethinyl estradiol tablets if there is an increased frequency or severity of migraines during COC use (which may be prodromal of a cerebrovascular event).
• Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive.• Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis [see].2. Liver DiseaseElevated Liver EnzymesLevonorgestrel and ethinyl estradiol tablets are contraindicated in females with acute viral hepatitis or severe (decompensated) cirrhosis of liver (see CONTRAINDICATIONS). Discontinue levonorgestrel and ethinyl estradiol tablets if jaundice develops. Acute liver test abnormalities may necessitate the discontinuation of COC use until the liver tests return to normal and COC causation has been excluded.
Liver TumorsLevonorgestrel and ethinyl estradiol tablets are contraindicated in females with benign or malignant liver tumors (see CONTRAINDICATIONS). COCs increase the risk of hepatic adenomas. An estimate of the attributable risk is 3.3 cases/100,000 COC users. Rupture of hepatic adenomas may cause death from abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (> 8 years) COC users. The attributable risk of liver cancers in COC users is less than one case per million users.
• Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations [see].5. Risk of Liver Enzyme Elevations with Concomitant Hepatitis C TreatmentDuring clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications such as COCs. Discontinue levonorgestrel and ethinyl estradiol tablets prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir (see CONTRAINDICATIONS). Levonorgestrel and ethinyl estradiol tablets can be restarted approximately 2 weeks following completion of treatment with the combination drug regimen.
• Undiagnosed abnormal uterine bleeding [see].9. Bleeding Irregularities and AmenorrheaUnscheduled Bleeding and SpottingFemales using levonorgestrel and ethinyl estradiol tablets may experience unscheduled (breakthrough or intracyclic) bleeding and spotting, especially during the first three months of use. Bleeding irregularities may resolve over time or by changing to a different contraceptive product. If bleeding persists or occurs after previously regular cycles, evaluate for causes such as pregnancy or malignancy.
In two clinical trials of levonorgestrel and ethinyl estradiol tablets (1084 subjects reporting for a total of 8186 treatment cycles and 238 subjects reporting for a total of 1102 treatment cycles), breakthrough bleeding occurred in 6.9% and 8.1% of reported cycles, and spotting occurred in 8.6% and 7.9% of reported cycles over the total study duration, respectively. In the two trials, intermenstrual bleeding (i.e., breakthrough bleeding and/or spotting) occurred in 13.1% and 12.9% of reported cycles over the total study duration, respectively. In one trial, 33 subjects out of 1084 (3.0%) discontinued due to bleeding irregularities (i.e., breakthrough bleeding and spotting); in the other trial, 6 subjects out of 238 (2.5%) discontinued due to bleeding irregularities.
Amenorrhea and OligomenorrheaFemales who use levonorgestrel and ethinyl estradiol tablets may experience absence of scheduled (withdrawal) bleeding, even if they are not pregnant. In two clinical trials of levonorgestrel and ethinyl estradiol tablets, one including 8186 reported treatment cycles, and the other including 1102 reported treatment cycles, amenorrhea occurred in 1.5% of treatment cycles in each trial.
If scheduled bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or two active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and perform appropriate diagnostic measures. If the patient has adhered to the prescribed dosing schedule and misses two consecutive periods, rule out pregnancy.
After discontinuation of a COC, amenorrhea or oligomenorrhea may occur, especially if these conditions were pre-existent.
The following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling:
• Serious cardiovascular adverse events [seeandWARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTSCigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs, including levonorgestrel and ethinyl estradiol tablets, are contraindicated in women who are over 35 years of age and smoke [see CONTRAINDICATIONSand WARNINGS (1)].
]1. Thromboembolic Disorders and Other Vascular Conditions• Stop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.• Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.• Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least four weeks before and through two weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.• Start levonorgestrel and ethinyl estradiol tablets no earlier than four weeks after delivery in females who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.• Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases (see CONTRAINDICATIONS).
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke (see CONTRAINDICATIONS). Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs (see CONTRAINDICATIONS). While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1 Likelihood of Developing a VTE


Fig 01 • Vascular events [see]1. Thromboembolic Disorders and Other Vascular Conditions• Stop levonorgestrel and ethinyl estradiol tablets if an arterial or venous thrombotic/thromboembolic event occurs.• Stop levonorgestrel and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.• Discontinue levonorgestrel and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets at least four weeks before and through two weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.• Start levonorgestrel and ethinyl estradiol tablets no earlier than four weeks after delivery in females who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.• Before starting levonorgestrel and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases (see CONTRAINDICATIONS).
Arterial EventsCOCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke (see CONTRAINDICATIONS). Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous EventsUse of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs (see CONTRAINDICATIONS). While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1 Likelihood of Developing a VTE


Fig 01 • Liver disease [see]2. Liver DiseaseElevated Liver EnzymesLevonorgestrel and ethinyl estradiol tablets are contraindicated in females with acute viral hepatitis or severe (decompensated) cirrhosis of liver (see CONTRAINDICATIONS). Discontinue levonorgestrel and ethinyl estradiol tablets if jaundice develops. Acute liver test abnormalities may necessitate the discontinuation of COC use until the liver tests return to normal and COC causation has been excluded.
Liver TumorsLevonorgestrel and ethinyl estradiol tablets are contraindicated in females with benign or malignant liver tumors (see CONTRAINDICATIONS). COCs increase the risk of hepatic adenomas. An estimate of the attributable risk is 3.3 cases/100,000 COC users. Rupture of hepatic adenomas may cause death from abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (> 8 years) COC users. The attributable risk of liver cancers in COC users is less than one case per million users.
• Hypertension [see]3. HypertensionLevonorgestrel and ethinyl estradiol tablets are contraindicated in females with uncontrolled hypertension or hypertension with vascular disease (see CONTRAINDICATIONS). For all females, including those with well-controlled hypertension, monitor blood pressure at routine visits and stop levonorgestrel and ethinyl estradiol tablets if blood pressure rises significantly.
An increase in blood pressure has been reported in females using COCs, and this increase is more likely in older women with extended duration of use. The effect of COCs on blood pressure may vary according to the progestin in the COC.
• Gallbladder disease [see]6. Gallbladder DiseaseStudies suggest an increased risk of developing gallbladder disease among COC users. Use of COCs may also worsen existing gallbladder disease.
A past history of COC-related cholestasis predicts an increased risk with subsequent COC use. Females with a history of pregnancy-related cholestasis may be at an increased risk for COC-related cholestasis.
• Carbohydrate and lipid effects [see]7. Adverse Carbohydrate and Lipid Metabolic EffectsHyperglycemiaLevonorgestrel and ethinyl estradiol tablets are contraindicated in diabetic women over age 35, or females who have diabetes with hypertension, nephropathy, retinopathy, neuropathy, other vascular disease, or females with diabetes of > 20 years duration (see CONTRAINDICATIONS). Levonorgestrel and ethinyl estradiol tablets may decrease glucose tolerance. Carefully monitor prediabetic and diabetic females who are using levonorgestrel and ethinyl estradiol tablets.
DyslipidemiaConsider alternative contraception for females with uncontrolled dyslipidemia. Levonorgestrel and ethinyl estradiol tablets may cause adverse lipid changes.
Females with hypertriglyceridemia, or a family history thereof, may have an increase in serum triglyceride concentrations when using levonorgestrel and ethinyl estradiol tablets which may increase the risk of pancreatitis.
• Headache [see]8. HeadacheLevonorgestrel and ethinyl estradiol tablets are contraindicated in females who have headaches with focal neurological symptoms or have migraine headaches with aura, and in women over age 35 years who have migraine headaches with or without aura (see CONTRAINDICATIONS).
If a woman using levonorgestrel and ethinyl estradiol tablets develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue levonorgestrel and ethinyl estradiol tablets if indicated. Consider discontinuation of levonorgestrel and ethinyl estradiol tablets if there is an increased frequency or severity of migraines during COC use (which may be prodromal of a cerebrovascular event).
• Carcinoma of the cervix [see WARNINGS (11)]
Adverse reactions reported by COC users and described elsewhere in the labeling are:
• Bleeding irregularities and amenorrhea [see]9. Bleeding Irregularities and AmenorrheaUnscheduled Bleeding and SpottingFemales using levonorgestrel and ethinyl estradiol tablets may experience unscheduled (breakthrough or intracyclic) bleeding and spotting, especially during the first three months of use. Bleeding irregularities may resolve over time or by changing to a different contraceptive product. If bleeding persists or occurs after previously regular cycles, evaluate for causes such as pregnancy or malignancy.
In two clinical trials of levonorgestrel and ethinyl estradiol tablets (1084 subjects reporting for a total of 8186 treatment cycles and 238 subjects reporting for a total of 1102 treatment cycles), breakthrough bleeding occurred in 6.9% and 8.1% of reported cycles, and spotting occurred in 8.6% and 7.9% of reported cycles over the total study duration, respectively. In the two trials, intermenstrual bleeding (i.e., breakthrough bleeding and/or spotting) occurred in 13.1% and 12.9% of reported cycles over the total study duration, respectively. In one trial, 33 subjects out of 1084 (3.0%) discontinued due to bleeding irregularities (i.e., breakthrough bleeding and spotting); in the other trial, 6 subjects out of 238 (2.5%) discontinued due to bleeding irregularities.
Amenorrhea and OligomenorrheaFemales who use levonorgestrel and ethinyl estradiol tablets may experience absence of scheduled (withdrawal) bleeding, even if they are not pregnant. In two clinical trials of levonorgestrel and ethinyl estradiol tablets, one including 8186 reported treatment cycles, and the other including 1102 reported treatment cycles, amenorrhea occurred in 1.5% of treatment cycles in each trial.
If scheduled bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or two active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and perform appropriate diagnostic measures. If the patient has adhered to the prescribed dosing schedule and misses two consecutive periods, rule out pregnancy.
After discontinuation of a COC, amenorrhea or oligomenorrhea may occur, especially if these conditions were pre-existent.
• Mood changes, including depression [see]10. DepressionCarefully observe females with a history of depression and discontinue levonorgestrel and ethinyl estradiol tablets if depression recurs to a serious degree. Data on the association of COCs with onset of depression or exacerbation of existing depression are limited.
• Melasma/chloasma which may persist [see]14. ChloasmaChloasma may occur with levonorgestrel and ethinyl estradiol tablets use, especially in females with a history of chloasma gravidarum. Advise females with a history of chloasma to avoid exposure to the sun or ultraviolet radiation while using levonorgestrel and ethinyl estradiol tablets.
• Edema/fluid retention [see]2. Fluid RetentionOral contraceptives may cause some degree of fluid retention. They should be prescribed with caution, and only with careful monitoring, in patients with conditions which might be aggravated by fluid retention.
• Diminution in lactation when given immediately postpartum [see]7. LactationRisk SummaryContraceptive hormones and/or metabolites are present in human milk. COCs can reduce milk production in breast-feeding females. This reduction can occur at any time but is less likely to occur once breast-feeding is well-established. When possible, advise the nursing female to use other methods of contraception until she discontinues breast-feeding. (see DOSAGE AND ADMINISTRATION). The developmental and health benefits of breast-feeding should be considered along with the mother’s clinical need for levonorgestrel and ethinyl estradiol tablets and any potential adverse effects on the breast-fed child from levonorgestrel and ethinyl estradiol tablets or from the underlying maternal condition.
Five studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 - 1.12 (Figure 2).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 2). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 - 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8-10 years of COC use.
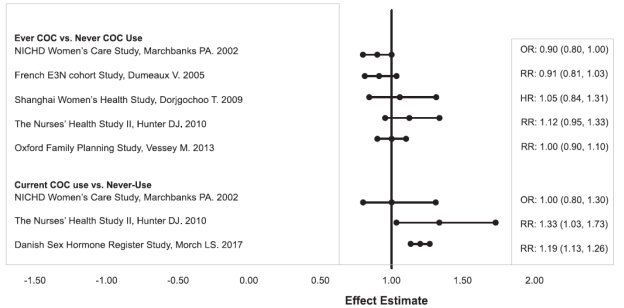
RR = relative risk; OR = odds ratio; HR = hazard ratio. “ever COC” are females with current or past COC use; “never COC use” are females that never used COCs.
The following adverse reactions have been reported in patients receiving oral contraceptives and are believed to be drug-related: Breast tenderness, pain, enlargement, secretion; Nausea, vomiting and gastrointestinal symptoms (such as abdominal pain, cramps and bloating); Change in menstrual flow; Temporary infertility after discontinuation of treatment; Change in weight or appetite (increase or decrease); Change in cervical erosion and secretion; Cholestatic jaundice; Rash (allergic); Vaginitis, including candidiasis; Change in corneal curvature (steepening); Intolerance to contact lenses; Mesenteric thrombosis; Decrease in serum folate levels; Exacerbation of systemic lupus erythematosus; Exacerbation of porphyria; Exacerbation of chorea; Aggravation of varicose veins; Anaphylactic/anaphylactoid reactions, including urticaria, angioedema, and severe reactions with respiratory and circulatory symptoms.
The following adverse reactions have been reported in users of oral contraceptives, and the association has been neither confirmed nor refuted: Congenital anomalies; Premenstrual syndrome; Cataracts; Optic neuritis, which may lead to partial or complete loss of vision; Cystitis-like syndrome; Nervousness; Dizziness; Hirsutism; Loss of scalp hair; Erythema multiforme; Erythema nodosum; Hemorrhagic eruption; Impaired renal function; Hemolytic uremic syndrome; Budd-Chiari syndrome; Acne; Changes in libido; Colitis; Sickle-cell disease; Cerebral-vascular disease with mitral valve prolapse; Lupus-like syndromes; Pancreatitis; Dysmenorrhea.
Levonorgestrel and ethinyl estradiol tablets USP, 0.15 mg/30 mcg are a combination oral contraceptives (COC) consisting of 21 white to off-white active tablets, each containing 0.15 mg of levonorgestrel, a synthetic progestin and 30 mcg of ethinyl estradiol, an estrogen, and 7 green inert tablets (without hormones).
The structural formulas for the active components are:
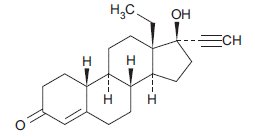
Levonorgestrel is chemically 18,19-Dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-,(17α)-(-)-.
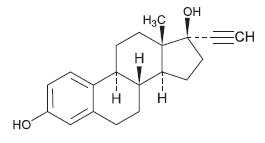
Ethinyl Estradiol is 19-Nor-17α-pregna-1,3,5(10)-trien-20-yne-3, 17-diol.
Each white to off-white active tablet contains the following inactive ingredients: lactose monohydrate, magnesium stearate, and polacrilin potassium.
Each green inert tablet contains the following inactive ingredients: FD&C Blue No. 1 aluminum lake, lactose monohydrate, magnesium stearate, polacrilin potassium, and yellow oxide of iron.