Lidocaine
(Lidocaine Hydrochloride)Lidocaine Prescribing Information
Lidocaine HCl 2% Jelly is indicated for prevention and control of pain in procedures involving the male and female urethra, for topical treatment of painful urethritis, and as an anesthetic lubricant for endotracheal intubation (oral and nasal).
When Lidocaine HCl 2% Jelly is used concomitantly with other products containing lidocaine, the total dose contributed by all formulations must be kept in mind.
The dosage varies and depends upon the area to be anesthetized, vascularity of the tissues, individual tolerance, and the technique of anesthesia. The lowest dosage needed to provide effective anesthesia should be administered. Dosages should be reduced for children and for elderly and debilitated patients. Although the incidence of adverse effects with Lidocaine HCl 2% Jelly is quite low, caution should be exercised, particularly when employing large amounts, since the incidence of adverse effects is directly proportional to the total dose of local anesthetic agent administered.
Lidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type or to other components of Lidocaine HCl 2% Jelly.
Adverse experiences following the administration of lidocaine are similar in nature to those observed in other amide local anesthetic agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage or rapid absorption, or may result from a hypersensitivity, idiosyncrasy, or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported:
There have been rare reports of endotracheal tube occlusion associated with the presence of dried jelly residue in the inner lumen of the tube (see
EXCESSIVE DOSAGE, OR SHORT INTERVALS BETWEEN DOSES, CAN RESULT IN HIGH PLASMA LEVELS AND SERIOUS ADVERSE EFFECTS. PATIENTS SHOULD BE INSTRUCTED TO STRICTLY ADHERE TO THE RECOMMENDED DOSAGE AND ADMINISTRATION GUIDELINES AS SET FORTH IN THIS PACKAGE INSERT. THE MANAGEMENT OF SERIOUS ADVERSE REACTIONS MAY REQUIRE THE USE OF RESUSCITATIVE EQUIPMENT, OXYGEN AND OTHER RESUSCITATIVE DRUGS.
Lidocaine HCl 2% Jelly should be used with extreme caution in the presence of sepsis or severely traumatized mucosa in the area of application, since under such conditions there is the potential for rapid systemic absorption.
When used for endotracheal tube lubrication care should be taken to avoid introducing the product into the lumen of the tube. Do not use the jelly to lubricate the endotracheal stylettes. If allowed into the inner lumen, the jelly may dry on the inner surface leaving a residue which tends to clump with flexion, narrowing the lumen. There have been rare reports in which this residue has caused the lumen to occlude (see
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue Lidocaine HCl 2% Jelly and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
When Lidocaine HCl 2% Jelly is used concomitantly with other products containing lidocaine, the total dose contributed by all formulations must be kept in mind.
The dosage varies and depends upon the area to be anesthetized, vascularity of the tissues, individual tolerance, and the technique of anesthesia. The lowest dosage needed to provide effective anesthesia should be administered. Dosages should be reduced for children and for elderly and debilitated patients. Although the incidence of adverse effects with Lidocaine HCl 2% Jelly is quite low, caution should be exercised, particularly when employing large amounts, since the incidence of adverse effects is directly proportional to the total dose of local anesthetic agent administered.
Prior to sounding or cystoscopy, a penile clamp should be applied for 5 to 10 minutes to obtain adequate anesthesia. A total dose of 30 mL (600 mg) is usually required to fill and dilate the male urethra. Prior to catheterization, smaller volumes of 5 to 10 mL (100 to 200 mg) are usually adequate for lubrication.
Drowsiness following the administration of lidocaine is usually an early sign of a high blood level of the drug and may occur as a consequence of rapid absorption.
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Class | Examples |
| Nitrates/Nitrites | nitric oxide, nitroglycerin, nitroprusside, nitrous oxide |
| Local anesthetics | articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine |
| Antineoplastics Agents | cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase |
| Antibiotics | dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides |
| Antimalarials | chloroquine, primaquine |
| Anticonvulsants | Phenobarbital, phenytoin, sodium valproate |
| Other drugs | acetaminophen, metoclopramide, quinine, sulfasalazine |
Lidocaine HCl 2% Jelly is a sterile aqueous product that contains a local anesthetic agent and is administered topically (see
Lidocaine HCl 2% Jelly is indicated for prevention and control of pain in procedures involving the male and female urethra, for topical treatment of painful urethritis, and as an anesthetic lubricant for endotracheal intubation (oral and nasal).
Lidocaine HCl 2% Jelly contains lidocaine HCl which is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, monohydrochloride and has the following structural formula:
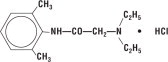
Its molecular formula is C
14H
22N
2O • HCl and its molecular weight is 270.80.
Lidocaine HCl 2% Jelly also contains hypromellose, and the resulting mixture maximizes contact with mucosa and provides lubrication for instrumentation. The unused portion should be discarded after initial use.
Composition of Lidocaine HCl 2% Jelly 30 mL and 5 mL tubes: Each mL contains 20 mg of lidocaine HCl. The formulation also contains methylparaben, propylparaben, hypromellose, and sodium hydroxide and/or hydrochloric acid to adjust pH to 6.0 to 7.0.