Lidocaine
Lidocaine Prescribing Information
Lidocaine Ointment 5% is indicated for production of anesthesia of accessible mucous membranes of the oropharynx.
It is also useful as an anesthetic lubricant for intubation and for the temporary relief of pain associated with minor burns, including sunburn, abrasions of the skin, and insect bites.
When Lidocaine Ointment 5% is used concomitantly with other products containing lidocaine, the total dose contributed by all formulations must be kept in mind.
A single application should not exceed 5 g of Lidocaine Ointment 5%, containing 250 mg of Lidocaine base (equivalent chemically to approximately 300 mg of lidocaine hydrochloride). This is roughly equivalent to squeezing a six (6) inch length of ointment from the tube. In a 70 kg adult this dose equals 3.6 mg/kg (1.6 mg/lb) lidocaine base. No more than one-half tube, approximately 17-20 g of ointment or 850-1000 mg lidocaine base, should be administered in any one day.
Although the incidence of adverse effects with Lidocaine Ointment 5% is quite low, caution should be exercised, particularly when employing large amounts, since the incidence of adverse effects is directly proportional to the total dose of local anesthetic agent administered.
It is difficult to recommend a maximum dose of any drug for children since this varies as a function of age and weight. For children less than ten years who have a normal lean body mass and a normal lean body development, the maximum dose may be determined by the application of one of the standard pediatric drug formulas (e.g., Clark's rule). For example a child of five years weighing 50 lbs., the dose of lidocaine should not exceed 75-100 mg when calculated according to Clark's rule. In any case, the maximum amount of lidocaine administered should not exceed 4.5 mg/kg (2 mg/lb) of body weight.
For medical use, apply topically for adequate control of symptoms. The use of a sterile gauze pad is suggested for application to broken skin tissue. Apply to the tube prior to intubation.
In dentistry, apply to previously dried oral mucosa. Subsequent removal of excess saliva with cotton rolls or saliva ejector minimizes dilution of the ointment, permits maximum penetration, and minimizes the possibility of swallowing the topical ointment.
For use in connection with the insertion of new dentures, apply to all denture surfaces contacting mucosa.
Lidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type or to other components of Lidocaine Ointment 5%.
Adverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage or rapid absorption, or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported:
CNS manifestations are excitatory and/or depressant and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest. The excitatory manifestations may be very brief or may not occur at all, in which case the first manifestation of toxicity may be drowsiness merging into unconsciousness and respiratory arrest. Drowsiness following the administration of lidocaine is usually an early sign of a high blood level of the drug and may occur as a consequence of rapid absorption.
Cardiovascular manifestations are usually depressant and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
Allergic reactions are characterized by cutaneous lesions, urticaria, edema or anaphylactoid reactions. Allergic reactions may occur as a result of sensitivity either to the local anesthetic agent or to other components in the formulation. Allergic reactions as a result of sensitivity to lidocaine are extremely rare and, if they occur, should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value.
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Class | Examples |
| Nitrates/Nitrites | nitric oxide, nitroglycerin, nitroprusside, nitrous oxide |
| Local anesthetics | articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine |
| Antineoplastic Agents | cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase |
| Antibiotics | dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides |
| Antimalarials | chloroquine, primaquine |
| Anticonvulsants | Phenobarbital, phenytoin, sodium valproate |
| Other drugs | acetaminophen, metoclopramide, quinine, sulfasalazine |
Lidocaine Ointment, USP 5% contains a local anesthetic agent and is administered topically. See INDICATIONS AND USAGE for specific uses.
Lidocaine Ointment, USP 5% contains lidocaine USP, which is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, and has the following structural formula:
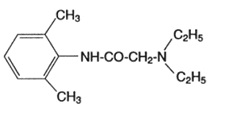
Composition of Lidocaine Ointment, USP 5%: acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, (lidocaine) 5% in a water miscible ointment vehicle containing polyethylene glycols.