Lopinavir And Ritonavir
Lopinavir And Ritonavir Prescribing Information
Contraindications (
- Lopinavir and ritonavir is contraindicated in patients with previously demonstrated clinically significant hypersensitivity (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome, erythema multiforme, urticaria, angioedema) to any of its ingredients, including ritonavir.
- Lopinavir and ritonavir is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening reactions[see Drug Interactions (7.1)and Clinical Pharmacology (12.3)].
○ Alpha 1- Adrenoreceptor Antagonist : alfuzosin
○ Antianginal: ranolazine
○ Antiarrhythmic: dronedarone
○ Anti-gout: colchicine
○ Antipsychotics: lurasidone, pimozide
○ Ergot Derivatives: dihydroergotamine, ergotamine, methylergonovine
○ GI Motility Agent: cisapride
○ Hepatitis C direct acting antiviral: elbasvir/grazoprevir
○ HMG-CoA Reductase Inhibitors: lovastatin, simvastatin
○ Microsomal triglyceride
○ PDE5 Inhibitor: sildenafil (Revatio®) when used for the treatment of pulmonary arterial hypertension
○ Sedative/Hypnotics: triazolam, orally administered midazolam
- Lopinavir and ritonavir is contraindicated with drugs that are potent CYP3A inducers where significantly reduced lopinavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance and cross-resistance[see Drug Interactions (7.2)and Clinical Pharmacology (12.3)].
○ Anticancer Agents: apalutamide
○ Antimycobacterial: rifampin
○ Herbal Products: St. John's Wort (hypericum perforatum)
- Hypersensitivity to lopinavir and ritonavir (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome, erythema multiforme, urticaria, angioedema) or any of its ingredients, including ritonavir.
- Co-administration with drugs highly dependent on CYP3A for clearance and for which elevated plasma levels may result in serious and/or life-threatening events.
- Co-administration with potent CYP3A inducers where significantly reduced lopinavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance and cross resistance.
Lopinavir and ritonavir is indicated in combination with other antiretroviral agents for the treatment of HIV- 1 infection in adults and pediatric patients 14 days and older.
Limitations of Use:
- Genotypic or phenotypic testing and/or treatment history should guide the use of lopinavir and ritonavir. The number of baseline lopinavir resistance-associated substitutions affects the virologic response to lopinavir and ritonavir [.]
12.4 MicrobiologyMechanism of ActionLopinavir, an inhibitor of the HIV-1 protease, prevents cleavage of the viral Gag-Pol polyprotein, resulting in the production of immature, non-infectious viral particles.
Antiviral ActivityIn the absence of human serum, the mean 50% effective concentration (EC50) values of lopinavir against five different HIV-1 subtype B laboratory strains in lymphoblastic cell lines ranged from 10 to 27 nM (0.006 to 0.017 mcg/mL, 1 mcg/mL = 1.6 mcM), and ranged from 4 to 11 nM (0.003 to 0.007 mcg/mL) against several HIV-1 subtype B clinical isolates in peripheral blood lymphocytes (n = 6). In the presence of 50% human serum, the mean EC50values of lopinavir against these five HIV-1 laboratory strains ranged from 65 to 289 nM (0.04 to 0.18 mcg/mL), representing a 7 to 11-fold attenuation. The EC50values of lopinavir against three different HIV-2 strains ranged from 12 to 180 nM (0.008 to 113 mcg/mL).
ResistanceHIV-1 isolates with reduced susceptibility to lopinavir have been selected in cell culture. The presence of ritonavir does not appear to influence the selection of lopinavir-resistant viruses in cell culture.
In a study of 653 antiretroviral treatment-naïve patients (Study 863), plasma viral isolates from each patient on treatment with plasma HIV-1 RNA >400 copies/mL at Week 24, 32, 40 and/or 48 were analyzed. No specific amino acid substitutions could be associated with resistance to lopinavir and ritonavir in the virus from 37 evaluable lopinavir and ritonavir-treated patients. The selection of resistance to lopinavir and ritonavir in antiretroviral treatment-naïve pediatric patients (Study 940) appears to be consistent with that seen in adult patients (Study 863).
Resistance to lopinavir and ritonavir has been noted to emerge in patients treated with other protease inhibitors prior to lopinavir and ritonavir therapy. In studies of 227 antiretroviral treatment-naïve and protease inhibitor experienced patients, isolates from 4 of 23 patients with quantifiable (>400 copies/mL) viral RNA following treatment with lopinavir and ritonavir for 12 to 100 weeks displayed significantly reduced susceptibility to lopinavir compared to the corresponding baseline viral isolates. All four of these patients had previously received treatment with at least one protease inhibitor and had at least 4 substitutions associated with protease inhibitor resistance immediately prior to lopinavir and ritonavir therapy. Following viral rebound, isolates from these patients all contained additional substitutions, some of which are recognized to be associated with protease inhibitor resistance.
Cross-resistance - Nonclinical StudiesVarying degrees of cross-resistance have been observed among HIV-1 protease inhibitors. The antiviral activity in cell culture of lopinavir against clinical isolates from patients previously treated with a single protease inhibitor was determined (Table 18).
Table 18. Susceptibility Reduction to Lopinavir Against Isolates from Patients Previously Treated With a Single Protease Inhibitor Susceptibility reduced by >4 foldSusceptibility reduced to LPVIndinavir (n=16) 5.7 fold Nelfinavir (n=13) <4 fold Ritonavir (n=3) 8.32 fold Saquinavir (n=4) <4 fold Isolates from patients previously treated with two or more protease inhibitors showed greater reductions in susceptibility to lopinavir, as described in the following section.
Clinical Studies - Antiviral Activity of Lopinavir and Ritonavir in Patients with Previous Protease Inhibitor TherapiesThe clinical relevance of reduced susceptibility in cell culture to lopinavir has been examined by assessing the virologic response to lopinavir and ritonavir therapy in treatment-experienced patients, with respect to baseline viral genotype in three studies and baseline viral phenotype in one study.
Virologic response to lopinavir and ritonavir has been shown to be affected by the presence of three or more of the following amino acid substitutions in protease at baseline: L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V, I54L/T/V, V82A/C/F/S/T, and I84V. Table 19 shows the 48-week virologic response (HIV-1 RNA <400 copies/mL) according to the number of the above protease inhibitor resistance-associated substitutions at baseline in studies 888 and 765
[ see Clinical Studies (14.2)and]and study 957 (see below). Once daily administration of lopinavir and ritonavir for adult patients with three or more of the above substitutions is not recommended.Table 19. Virologic Response (HIV-1 RNA <400 copies/mL) at Week 48 by Baseline Lopinavir and Ritonavir Susceptibility and by Number of Protease Substitutions Associated with Reduced Response to Lopinavir and Ritonavir1 Number of protease inhibitor substitutions at baseline1Study 888 (Single protease inhibitor-experienced2, NNRTI-naïve) n=130Study 765 (Single protease inhibitor-experienced3, NNRTI-naïve) n=56Study 957 (Multiple protease inhibitor-experienced4, NNRTI-naïve) n=500 to 2 76/103 (74%) 34/45 (76%) 19/20 (95%) 3 to 5 13/26 (50%) 8/11 (73%) 18/26 (69%) 6 or more 0/1 (0%) N/A 1/4 (25%) 1Substitutions considered in the analysis included L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V, I54L/T/V, V82A/C/F/S/T, and I84V.
243% indinavir, 42% nelfinavir, 10% ritonavir, 15% saquinavir.
341% indinavir, 38% nelfinavir, 4% ritonavir, 16% saquinavir.
486% indinavir, 54% nelfinavir, 80% ritonavir, 70% saquinavir.Virologic response to lopinavir and ritonavir therapy with respect to phenotypic susceptibility to lopinavir at baseline was examined in Study 957. In this study 56 NNRTI-naïve patients with HIV-1 RNA >1,000 copies/mL despite previous therapy with at least two protease inhibitors selected from indinavir, nelfinavir, ritonavir, and saquinavir were randomized to receive one of two doses of lopinavir and ritonavir in combination with efavirenz and nucleoside reverse transcriptase inhibitors (NRTIs). The EC50values of lopinavir against the 56 baseline viral isolates ranged from 0.5- to 96-fold the wild-type EC50value. Fifty-five percent (31/56) of these baseline isolates displayed >4-fold reduced susceptibility to lopinavir. These 31 isolates had a median reduction in lopinavir susceptibility of 18-fold. Response to therapy by baseline lopinavir susceptibility is shown in Table 20.
Table 20. HIV-1 RNA Response at Week 48 by Baseline Lopinavir Susceptibility1 Lopinavir susceptibility2at baselineHIV-1 RNA <400 copies/mL(%)HIV-1 RNA <50 copies/mL(%)< 10 fold 25/27 (93%) 22/27 (81%) > 10 and < 40 fold 11/15 (73%) 9/15 (60%) ≥ 40 fold 2/8 (25%) 2/8 (25%) 1Lopinavir susceptibility was determined by recombinant phenotypic technology performed by Virologic.
2Fold change in susceptibility from wild type.
Tablets: May be taken with or without food, swallowed whole and not chewed, broken, or crushed. (
Lopinavir and ritonavir tablets may be taken with or without food. The tablets should be swallowed whole and not chewed, broken, or crushed. Lopinavir and ritonavir oral solution must be taken with food.
Oral solution: must be taken with food. (
Lopinavir and ritonavir tablets may be taken with or without food. The tablets should be swallowed whole and not chewed, broken, or crushed. Lopinavir and ritonavir oral solution must be taken with food.
- Adult patients with three or more of the following lopinavir resistance-associated substitutions: L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V, I54L/T/V, V82A/C/F/S/T, and I84V[ see Microbiology (12.4)].
- In combination with carbamazepine, phenobarbital, or phenytoin[ see Drug Interactions (7.3)].
- In combination with efavirenz, nevirapine, or nelfinavir[ see Drug Interactions (7.3)and Clinical Pharmacology (12.3)].
- In pediatric patients younger than 18 years of age[ see Dosage and Administration (2.4)].
- In pregnant women[ see Dosage and Administration (2.5), Use in Specific Populations (8.1)and Clinical Pharmacology (12.3)].
Lopinavir and Ritonavir Dosage Form | Recommended Dosage |
| 200 mg/50 mg Tablets | 800 mg/200 mg (4 tablets) once daily |
| 80 mg/20 mg per mL Oral Solution | 800 mg/200 mg (10 mL) once daily |
Lopinavir and Ritonavir Dosage Form | Recommended Dosage |
| 200 mg/50 mg Tablets | 400 mg/100 mg (2 tablets) twice daily |
| 80 mg/20 mg per mL Oral Solution | 400 mg/100 mg (5 mL) twice daily |
The dose of lopinavir and ritonavir must be increased when administered in combination with efavirenz, nevirapine or nelfinavir. Table 3 outlines the dosage recommendations for twice daily dosing when lopinavir and ritonavir is taken in combination with these agents.
Lopinavir and Ritonavir Dosage Form | Recommended Dosage |
| 200 mg/50 mg Tablets and 100 mg/25 mg Tablets | 500 mg/125 mg (2 tablets of 200 mg/50 mg + 1 tablet of 100 mg/25 mg) twice daily |
| 80 mg/20 mg per mL Oral Solution | 520 mg/130 mg (6.5 mL) twice daily |
- Total recommended daily dosage is 800/200 mg given once or twice daily.
- Lopinavir and ritonavir can be given as once daily or twice daily regimen. See Full Prescribing Information for details.
- Lopinavir and ritonavir once daily dosing regimen is not recommended in:
- Adult patients with three or more of the following lopinavir resistance-associated substitutions: L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V, I54L/T/V, V82A/C/F/S/T, and I84V. ()
12.4 MicrobiologyMechanism of ActionLopinavir, an inhibitor of the HIV-1 protease, prevents cleavage of the viral Gag-Pol polyprotein, resulting in the production of immature, non-infectious viral particles.
Antiviral ActivityIn the absence of human serum, the mean 50% effective concentration (EC50) values of lopinavir against five different HIV-1 subtype B laboratory strains in lymphoblastic cell lines ranged from 10 to 27 nM (0.006 to 0.017 mcg/mL, 1 mcg/mL = 1.6 mcM), and ranged from 4 to 11 nM (0.003 to 0.007 mcg/mL) against several HIV-1 subtype B clinical isolates in peripheral blood lymphocytes (n = 6). In the presence of 50% human serum, the mean EC50values of lopinavir against these five HIV-1 laboratory strains ranged from 65 to 289 nM (0.04 to 0.18 mcg/mL), representing a 7 to 11-fold attenuation. The EC50values of lopinavir against three different HIV-2 strains ranged from 12 to 180 nM (0.008 to 113 mcg/mL).
ResistanceHIV-1 isolates with reduced susceptibility to lopinavir have been selected in cell culture. The presence of ritonavir does not appear to influence the selection of lopinavir-resistant viruses in cell culture.
In a study of 653 antiretroviral treatment-naïve patients (Study 863), plasma viral isolates from each patient on treatment with plasma HIV-1 RNA >400 copies/mL at Week 24, 32, 40 and/or 48 were analyzed. No specific amino acid substitutions could be associated with resistance to lopinavir and ritonavir in the virus from 37 evaluable lopinavir and ritonavir-treated patients. The selection of resistance to lopinavir and ritonavir in antiretroviral treatment-naïve pediatric patients (Study 940) appears to be consistent with that seen in adult patients (Study 863).
Resistance to lopinavir and ritonavir has been noted to emerge in patients treated with other protease inhibitors prior to lopinavir and ritonavir therapy. In studies of 227 antiretroviral treatment-naïve and protease inhibitor experienced patients, isolates from 4 of 23 patients with quantifiable (>400 copies/mL) viral RNA following treatment with lopinavir and ritonavir for 12 to 100 weeks displayed significantly reduced susceptibility to lopinavir compared to the corresponding baseline viral isolates. All four of these patients had previously received treatment with at least one protease inhibitor and had at least 4 substitutions associated with protease inhibitor resistance immediately prior to lopinavir and ritonavir therapy. Following viral rebound, isolates from these patients all contained additional substitutions, some of which are recognized to be associated with protease inhibitor resistance.
Cross-resistance - Nonclinical StudiesVarying degrees of cross-resistance have been observed among HIV-1 protease inhibitors. The antiviral activity in cell culture of lopinavir against clinical isolates from patients previously treated with a single protease inhibitor was determined (Table 18).
Table 18. Susceptibility Reduction to Lopinavir Against Isolates from Patients Previously Treated With a Single Protease Inhibitor Susceptibility reduced by >4 foldSusceptibility reduced to LPVIndinavir (n=16) 5.7 fold Nelfinavir (n=13) <4 fold Ritonavir (n=3) 8.32 fold Saquinavir (n=4) <4 fold Isolates from patients previously treated with two or more protease inhibitors showed greater reductions in susceptibility to lopinavir, as described in the following section.
Clinical Studies - Antiviral Activity of Lopinavir and Ritonavir in Patients with Previous Protease Inhibitor TherapiesThe clinical relevance of reduced susceptibility in cell culture to lopinavir has been examined by assessing the virologic response to lopinavir and ritonavir therapy in treatment-experienced patients, with respect to baseline viral genotype in three studies and baseline viral phenotype in one study.
Virologic response to lopinavir and ritonavir has been shown to be affected by the presence of three or more of the following amino acid substitutions in protease at baseline: L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V, I54L/T/V, V82A/C/F/S/T, and I84V. Table 19 shows the 48-week virologic response (HIV-1 RNA <400 copies/mL) according to the number of the above protease inhibitor resistance-associated substitutions at baseline in studies 888 and 765
[ see Clinical Studies (14.2)and]and study 957 (see below). Once daily administration of lopinavir and ritonavir for adult patients with three or more of the above substitutions is not recommended.Table 19. Virologic Response (HIV-1 RNA <400 copies/mL) at Week 48 by Baseline Lopinavir and Ritonavir Susceptibility and by Number of Protease Substitutions Associated with Reduced Response to Lopinavir and Ritonavir1 Number of protease inhibitor substitutions at baseline1Study 888 (Single protease inhibitor-experienced2, NNRTI-naïve) n=130Study 765 (Single protease inhibitor-experienced3, NNRTI-naïve) n=56Study 957 (Multiple protease inhibitor-experienced4, NNRTI-naïve) n=500 to 2 76/103 (74%) 34/45 (76%) 19/20 (95%) 3 to 5 13/26 (50%) 8/11 (73%) 18/26 (69%) 6 or more 0/1 (0%) N/A 1/4 (25%) 1Substitutions considered in the analysis included L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V, I54L/T/V, V82A/C/F/S/T, and I84V.
243% indinavir, 42% nelfinavir, 10% ritonavir, 15% saquinavir.
341% indinavir, 38% nelfinavir, 4% ritonavir, 16% saquinavir.
486% indinavir, 54% nelfinavir, 80% ritonavir, 70% saquinavir.Virologic response to lopinavir and ritonavir therapy with respect to phenotypic susceptibility to lopinavir at baseline was examined in Study 957. In this study 56 NNRTI-naïve patients with HIV-1 RNA >1,000 copies/mL despite previous therapy with at least two protease inhibitors selected from indinavir, nelfinavir, ritonavir, and saquinavir were randomized to receive one of two doses of lopinavir and ritonavir in combination with efavirenz and nucleoside reverse transcriptase inhibitors (NRTIs). The EC50values of lopinavir against the 56 baseline viral isolates ranged from 0.5- to 96-fold the wild-type EC50value. Fifty-five percent (31/56) of these baseline isolates displayed >4-fold reduced susceptibility to lopinavir. These 31 isolates had a median reduction in lopinavir susceptibility of 18-fold. Response to therapy by baseline lopinavir susceptibility is shown in Table 20.
Table 20. HIV-1 RNA Response at Week 48 by Baseline Lopinavir Susceptibility1 Lopinavir susceptibility2at baselineHIV-1 RNA <400 copies/mL(%)HIV-1 RNA <50 copies/mL(%)< 10 fold 25/27 (93%) 22/27 (81%) > 10 and < 40 fold 11/15 (73%) 9/15 (60%) ≥ 40 fold 2/8 (25%) 2/8 (25%) 1Lopinavir susceptibility was determined by recombinant phenotypic technology performed by Virologic.
2Fold change in susceptibility from wild type. - In combination with carbamazepine, phenobarbital, or phenytoin. ()
7.3 Established and Other Potentially Significant Drug InteractionsTable 12 provides a listing of established or potentially clinically significant drug interactions. Alteration in dose or regimen may be recommended based on drug interaction studies or predicted interaction
[see Contraindications (4), Warnings and Precautions (5.1), Clinical Pharmacology (12.3)]for magnitude of interaction.Table 12. Established and Other Potentially Significant Drug Interactions Concomitant Drug Class:Drug NameEffect on Concentration of Lopinavir or Concomitant DrugClinical CommentsHIV-1 Antiviral AgentsHIV-1 Protease Inhibitor: fosamprenavir/ritonavir ↓ amprenavir
↓ lopinavirAn increased rate of adverse reactions has been observed with co-administration of these medications. Appropriate doses of the combinations with respect to safety and efficacy have not been established. HIV-1 Protease Inhibitor: indinavir* ↑ indinavir Decrease indinavir dose to 600 mg twice daily, when co-administered with lopinavir and ritonavir 400/100 mg twice daily. Lopinavir and ritonavir once daily has not been studied in combination with indinavir. HIV-1 Protease Inhibitor: nelfinavir* ↑ nelfinavir
↑ M8 metabolite of nelfinavir
↓ lopinavirLopinavir and ritonavir once daily in combination with nelfinavir is not recommended [see Dosage and Administration (2)].HIV-1 Protease Inhibitor: ritonavir* ↑ lopinavir Appropriate doses of additional ritonavir in combination with lopinavir and ritonavir with respect to safety and efficacy have not been established. HIV-1 Protease Inhibitor: saquinavir ↑ saquinavir The saquinavir dose is 1,000 mg twice daily, when co-administered with lopinavir and ritonavir 400/100 mg twice daily. Lopinavir and ritonavir once daily has not been studied in combination with saquinavir. HIV-1 Protease Inhibitor: tipranavir* ↓ lopinavir Co-administration with tipranavir (500 mg twice daily) and ritonavir (200 mg twice daily) is not recommended. HIV CCR5 – Antagonist: maraviroc* ↑ maraviroc When co-administered, patients should receive 150 mg twice daily of maraviroc. For further details see complete prescribing information for maraviroc. Non-nucleoside Reverse Transcriptase Inhibitors: efavirenz*,
nevirapine*↓ lopinavir Increase the dose of lopinavir and ritonavir tablets to 500/125 mg when lopinavir and ritonavir tablet is co-administered with efavirenz or nevirapine. Lopinavir and ritonavir once daily in combination with efavirenz or nevirapine is not recommended [see Dosage and Administration (2)].Non-nucleoside Reverse Transcriptase Inhibitor: delavirdine ↑ lopinavir Appropriate doses of the combination with respect to safety and efficacy have not been established. Nucleoside Reverse Transcriptase Inhibitor: didanosine Lopinavir and ritonavir tablets can be administered simultaneously with didanosine without food.
For lopinavir and ritonavir oral solution, it is recommended that didanosine be administered on an empty stomach; therefore, didanosine should be given one hour before or two hours after lopinavir and ritonavir oral solution (given with food).Nucleoside Reverse Transcriptase Inhibitor: tenofovir disoproxil fumarate* ↑ tenofovir Patients receiving lopinavir and ritonavir and tenofovir should be monitored for adverse reactions associated with tenofovir. Nucleoside Reverse Transcriptase Inhibitors: abacavir
zidovudine↓ abacavir
↓ zidovudineThe clinical significance of this potential interaction is unknown. Other AgentsAlpha 1- Adrenoreceptor
Antagonist:
alfuzosin↑ alfuzosin Contraindicated due to potential hypotension [see Contraindications (4)].Antianginal:
ranolazine↑ ranolazine Contraindicated due to potential for serious and/or life-threatening reactions [see.Contraindications (4)]Antiarrhythmics:
dronedarone↑ dronedarone Contraindicated due to potential for cardiac arrhythmias [seeContraindications (4)].Antiarrhythmics e.g. amiodarone,
bepridil,
lidocaine (systemic),
quinidine↑ antiarrhythmics Caution is warranted and therapeutic concentration monitoring (if available) is recommended for antiarrhythmics when co-administered with lopinavir and ritonavir. Anticancer Agents:
abemaciclib,
apalutamide,
encorafenib,
ibrutinib,
ivosidenib,
dasatinib,
neratinib,
nilotinib,
venetoclax,
vinblastine,
vincristine↑ anticancer agents
↓lopinavir/ritonavir#Apalutamide is contraindicated due to potential for loss of virologic response and possible resistance to lopinavir and ritonavir or to the class of protease inhibitors [seeContraindications (4)].
Avoid co-administration of encorafenib or ivosidenib with lopinavir and ritonavir due to potential risk of serious adverse events such as QT interval prolongation. If co-administration of encorafenib with lopinavir and ritonavir cannot be avoided, modify dose as recommended in encorafenib USPI. If co-administration of ivosidenib with lopinavir and ritonavir cannot be avoided, reduce ivosidenib dose to 250 mg once daily.Avoid use of neratinib, venetoclax or ibrutinib with lopinavir and ritonavir.
For vincristine and vinblastine, consideration should be given to temporarily withholding the ritonavir-containing antiretroviral regimen in patients who develop significant hematologic or gastrointestinal side effects when lopinavir and ritonavir is administered concurrently with vincristine or vinblastine. If the antiretroviral regimen must be withheld for a prolonged period, consideration should be given to initiating a revised regimen that does not include a CYP3A or P-gp inhibitor.A decrease in the dosage or an adjustment of the dosing interval of nilotinib and dasatinib may be necessary for patients requiring co-administration with strong CYP3A inhibitors such as lopinavir and ritonavir. Please refer to the nilotinib and dasatinib prescribing information for dosing instructions. Anticoagulants:
warfarin,
rivaroxaban↑↓ warfarin
↑ rivaroxabanConcentrations of warfarin may be affected. Initial frequent monitoring of the INR during lopinavir and ritonavir and warfarin co-administration is recommended.
Avoid concomitant use of rivaroxaban and lopinavir and ritonavir. Co-administration of lopinavir and ritonavir and rivaroxaban may lead to increased risk of bleeding.Anticonvulsants:
carbamazepine,
phenobarbital,
phenytoin↓ lopinavir
↓ phenytoinLopinavir and ritonavir may be less effective due to decreased lopinavir plasma concentrations in patients taking these agents concomitantly and should be used with caution.
Lopinavir and ritonavir once daily in combination with carbamazepine, phenobarbital, or phenytoin is not recommended.
In addition, co-administration of phenytoin and lopinavir and ritonavir may cause decreases in steady-state phenytoin concentrations. Phenytoin levels should be monitored when co-administering with lopinavir and ritonavir.Anticonvulsants: lamotrigine,
valproate↓ lamotrigine
↓ or ↔ valproateA dose increase of lamotrigine or valproate may be needed when co-administered with lopinavir and ritonavir and therapeutic concentration monitoring for lamotrigine may be indicated; particularly during dosage adjustments. Antidepressant: bupropion ↓ bupropion
↓ active metabolite,
hydroxybupropionPatients receiving lopinavir and ritonavir and bupropion concurrently should be monitored for an adequate clinical response to bupropion. Antidepressant: trazodone ↑ trazodone Adverse reactions of nausea, dizziness, hypotension and syncope have been observed following co-administration of trazodone and ritonavir. A lower dose of trazodone should be considered. Anti-infective: clarithromycin ↑ clarithromycin For patients with renal impairment, adjust clarithromycin dose as follows: - For patients on lopinavir and ritonavir with CLCR30 to 60 mL/min the dose of clarithromycin should be reduced by 50%.
- For patients on lopinavir and ritonavir with CLCR< 30 mL/min the dose of clarithromycin should be decreased by 75%.
No dose adjustment for patients with normal renal function is necessary.Antifungals: ketoconazole*, itraconazole, voriconazole isavuconazonium sulfate* ↑ ketoconazole
↑ itraconazole
↓ voriconazole
↑ isavuconazoniumHigh doses of ketoconazole (>200 mg/day) or itraconazole (> 200 mg/day) are not recommended.
The coadministration of voriconazole and lopinavir and ritonavir should be avoided unless an assessment of the benefit/risk to the patient justifies the use of voriconazole.
Isavuconazonium and lopinavir and ritonavir should be coadministered with caution. Alternative antifungal therapies should be considered in these patients.Anti-gout: colchicine ↑ colchicine Contraindicated due to potential for serious and/or life-threatening reactions in patients with renal and/or hepatic impairment [see.Contraindications (4)]For patients with normal renal or hepatic function:Treatment of gout flares-co-administration of colchicine in patients onlopinavir and ritonavir:
0.6 mg (1 tablet) x 1 dose, followed by 0.3 mg (half tablet) 1 hour later. Dose to be repeated no earlier than 3 days.Prophylaxis of gout flares-co-administration of colchicine in patients onlopinavir and ritonavir:
If the original colchicine regimen was 0.6 mg twice a day, the regimen should be adjusted to 0.3 mg once a day.If the original colchicine regimen was 0.6 mg once a day, the regimen should be adjusted to 0.3 mg once every other day. Treatment of familial Mediterranean fever (FMF)-co-administration of colchicine in patients onlopinavir and ritonavir:
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day).Antimycobacterial:
rifampin↓ lopinavir Contraindicated due to potential loss of virologic response and possible resistance to lopinavir and ritonavir or to the class of protease inhibitors or other co-administered antiretroviral agents [seeContraindications (4)].Antimycobacterial: bedaquiline ↑ bedaquiline Bedaquiline should only be used with lopinavir and ritonavirif the benefit of co-administration outweighs the risk. Antimycobacterial: rifabutin* ↑ rifabutin and rifabutin metabolite Dosage reduction of rifabutin by at least 75% of the usual dose of 300 mg/day is recommended (i.e., a maximum dose of 150 mg every other day or three times per week). Increased monitoring for adverse reactions is warranted in patients receiving the combination. Further dosage reduction of rifabutin may be necessary. Antiparasitic: atovaquone ↓ atovaquone Clinical significance is unknown; however, increase in atovaquone doses may be needed. Antipsychotics:
lurasidone
pimozide↑ lurasidone
↑ pimozideContraindicated due to potential for serious and/or life-threatening reactions [seeContraindications (4)].
Contraindicated due to potential for serious and/or life-threatening reactions such as cardiac
arrhythmias[seeContraindications (4)].Antipsychotics: quetiapine ↑ quetiapine Initiation of lopinavir and ritonavir in patients taking quetiapine:
Consider alternative antiretroviral therapy to avoid increases in quetiapine exposures. If coadministration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring.Initiation of quetiapine in patients taking lopinavir and ritonavir:
Refer to the quetiapine prescribing information for initial dosing and titration of quetiapine.Contraceptive:
ethinyl estradiol*↓ ethinyl estradiol Because contraceptive steroid concentrations may be altered when lopinavir and ritonavir is co- administered with oral contraceptives or with the contraceptive patch, alternative methods of nonhormonal contraception are recommended. Dihydropyridine Calcium
Channel Blockers: e.g.
felodipine,
nifedipine,
nicardipine↑ dihydropyridine
calcium channel
blockersClinical monitoring of patients is recommended and a dose reduction of the dihydropyridine calcium channel blocker may be considered. Disulfiram/metronidazole Lopinavir and ritonavir oral solution contains ethanol, which can produce disulfiram-like reactions when co-administered with disulfiram or other drugs that produce this reaction (e.g., metronidazole). Endothelin Receptor Antagonists: bosentan ↑ bosentan Co-administration of bosentan in patients on lopinavir and ritonavir:
In patients who have been receiving lopinavir and ritonavir for at least 10 days, start bosentan at 62.5 mg once daily or every other day based upon individual tolerability.Co-administration of lopinavir and ritonavir in patients on bosentan:
Discontinue use of bosentan at least 36 hours prior to initiation of lopinavir and ritonavir.
After at least 10 days following the initiation of lopinavir and ritonavir, resume bosentan at 62.5 mg once daily or every other day based upon individual tolerability.Ergot Derivatives:
dihydroergotamine,
ergotamine,
methylergonovine↑ ergot derivatives Contraindicated due to potential for acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues [seeContraindications (4)].GI Motility Agent:
cisapride↑ cisapride Contraindicated due to potential for cardiac arrhythmias [seeContraindications (4)].GnRH Receptor Antagonists: elagolix ↑ elagolix
↓ lopinavir/ritonavirConcomitant use of elagolix 200 mg twice daily and lopinavir and ritonavir for more than 1 month is not recommended due to potential risk of adverse events such as bone loss and hepatic transaminase elevations. Limit concomitant use of elagolix 150 mg once daily and lopinavir and ritonavir to 6 months. Hepatitis C direct acting antiviral: elbasvir/grazoprevir ↑ elbasvir/grazoprevir Contraindicated due to increased risk of alanine transaminase (ALT) elevations [seeContraindications (4)].Hepatitis C direct acting antivirals:
boceprevir*
glecaprevir/Pibrentasvir
simeprevir
sofosbuvir/velpatasvir/voxilaprevi
r
ombitasvir/paritaprevir/ ritonavir and dasabuvir*↓ lopinavir
↓ boceprevir
↓ ritonavir
↑ glecaprevir
↑ pibrentasvir
↑ simeprevir
↑ sofosbuvir
↑ velpatasvir
↑ voxilaprevir
↑ ombitasvir
↑ paritaprevir
↑ ritonavir
↔ dasabuvirIt is not recommended to co-administer lopinavir and ritonavir and boceprevir, glecaprevir/pibrentasvir, simeprevir, sofosbuvir/velpatasvir/voxilaprevir,or ombitasvir/paritaprevir/ritonavir and dasabuvir. Herbal Products:
St. John's Wort (hypericum
perforatum)↓ lopinavir Contraindicated due to potential for loss of virologic response and possible resistance to lopinavir and ritonavir or to the class of protease inhibitors [seeContraindications (4)].Lipid-modifying agents
HMG-CoA Reductase Inhibitors:
lovastatin
simvastatin
atorvastatin
rosuvastatin
Microsomal triglyceride transfer
protein (MTTP) Inhibitor:
lomitapide
↑ lovastatin
↑ simvastatin
↑ atorvastatin
↑ rosuvastatin
↑ lomitapide
Contraindicated due to potential for myopathy including rhabdomyolysis[seeContraindications (4)].
Use atorvastatin with caution and at the lowest necessary dose. Titrate rosuvastatin dose carefully and use the lowest necessary dose; do not exceed rosuvastatin 10 mg/day.
Lomitapide is a sensitive substrate for CYP3A4 metabolism. CYP3A4 inhibitors increase the
exposure of lomitapide, with strong inhibitors increasing exposure approximately 27-fold.
Concomitant use of moderate or strong CYP3A4 inhibitors with lomitapide is contraindicated due to potential for hepatotoxicity[see.Contraindications (4)]Immunosuppressants: e.g.
cyclosporine,
tacrolimus,
sirolimus↑ immunosuppressants Therapeutic concentration monitoring is recommended for immunosuppressant agents when co-administered with lopinavir and ritonavir. Kinase Inhibitors:
fostamatinib(also see anticancer agents above)↑ fostamatinib
metabolite R406Monitor for toxicities of R406 such as hepatotoxicity and neutropenia. Fostamatinib dose reduction may be required. Long-acting beta-adrenoceptor Agonist:
salmeterol↑ salmeterol Concurrent administration of salmeterol and lopinavir and ritonavir is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations and sinus tachycardia. Narcotic Analgesics: methadone,*
fentanyl↓ methadone
↑ fentanylDosage of methadone may need to be increased when co-administered with lopinavir and ritonavir.
Careful monitoring of therapeutic and adverse effects (including potentially fatal respiratory depression) is recommended when fentanyl is concomitantly administered with lopinavir and ritonavir.PDE5 inhibitors: avanafil,
sildenafil,
tadalafil,
vardenafil↑ avanafil
↑ sildenafil
↑ tadalafil
↑ vardenafilSildenafil when used for the treatment of pulmonary arterial hypertension (Revatio®) is contraindicated due to the potential for sildenafil-associated adverse events, including visual abnormalities, hypotension, prolonged erection, and syncope [see.Contraindications(4)]
Do not use lopinavir and ritonavir with avanafil because a safe and effective avanafil dosage regimen has not been established.
Particular caution should be used when prescribing sildenafil, tadalafil, or vardenafil in patients receiving lopinavir and ritonavir. Co-administration of lopinavir and ritonavir with these drugs may result in an increase in PDE5 inhibitor associated adverse reactions including hypotension, syncope, visual changes and prolonged erection.
Use of PDE5 inhibitors for pulmonary arterial hypertension (PAH):
Sildenafil (Revatio®) is contraindicated[see.Contraindications (4)]
The following dose adjustments are recommended for use of tadalafil (Adcirca®) with lopinavir and ritonavir:Co-administration of ADCIRCA in patients on lopinavir and ritonavir:
In patients receiving lopinavir and ritonavir for at least one week, start ADCIRCA at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability.Co-administration of lopinavir and ritonavir in patients on ADCIRCA:
Avoid use of ADCIRCA during the initiation of lopinavir and ritonavir. Stop ADCIRCA at least 24 hours prior to starting lopinavir and ritonavir. After at least one week following the initiation of lopinavir and ritonavir, resume ADCIRCA at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability.
Use of PDE5 inhibitors for erectile dysfunction:
It is recommended not to exceed the following doses:- Sildenafil: 25 mg every 48 hours
- Tadalafil: 10 mg every 72 hours
- Vardenafil: 2.5 mg every 72 hours
Sedative/Hypnotics:
triazolam,
orally administered midazolam↑ triazolam
↑ midazolamContraindicated due to potential for prolonged or increased sedation or respiratory depression [seeContraindications (4)].Sedative/Hypnotics:
parenterally administered midazolam↑ midazolam If lopinavir and ritonavir is co-administered with parenteral midazolam, close clinical monitoring for respiratory depression and/or prolonged sedation should be exercised and dosage adjustment should be considered. Systemic/Inhaled/
Nasal/Ophthalmic
Corticosteroids: e.g.,
betamethasone
budesonide
ciclesonide
dexamethasone
fluticasone
methylprednisolone
mometasone
prednisone
triamcinolone↓ lopinavir
↑ glucocorticoidsCoadministration with oral dexamethasone or other systemic corticosteroids that induce CYP3A may result in loss of therapeutic effect and development of resistance to lopinavir. Consider alternative corticosteroids.
Coadministration with corticosteroids whose exposures are significantly increased by strong CYP3A inhibitors can increase the risk for Cushing’s syndrome and adrenal suppression.
Alternative corticosteroids including beclomethasone and prednisolone (whose PK and/or PD are less affected by strong CYP3A inhibitors relative to other studied steroids) should be considered, particularly for long-term use.* seefor magnitude of interaction.Clinical Pharmacology (12.3)
#refers to interaction with apalutamide. - In combination with efavirenz, nevirapine, or nelfinavir. ()
12.3 PharmacokineticsThe pharmacokinetic properties of lopinavir are summarized in Table 13. The steady-state pharmacokinetic parameters of lopinavir are summarized in Table 14. Under fed conditions, lopinavir concentrations were similar following administration of lopinavir and ritonavir tablets to capsules with less pharmacokinetic variability. Under fed conditions (500 kcal, 25% from fat), lopinavir concentrations were similar following administration of lopinavir and ritonavir capsules and oral solution.
Table 13. Pharmacokinetic Properties of Lopinavir AbsorptionTmax(hr)a 4.4 ± 0.8 Effect of meal
(relative to fasting)
Tablet
Oral solution
↑ 19%b
↑ 130%bDistribution% Bound to human plasma proteins > 98 Vd/Fa(L) 16.9 MetabolismMetabolism CYP3A EliminationMajor route of elimination hepatic t1/2(h)a 6.9 ± 2.2 % of dose excreted in urine 10.4 ± 2.3 % of dose excreted in feces 82.6 ± 2.5 a.Lopinavir and ritonavir tablet
b.Changes in AUC valuesTable 14. Steady-State Pharmacokinetic Parameters of Lopinavir, Mean ± SD Pharmacokinetic ParameterTwice DailyaOnce DailybCmax(mcg/mL) 9.8 ± 3.7 11.8 ± 3.7 Cmin(mcg/mL) 5.5 ± 2.7 1.7 ± 1.6 AUCtau(mcg•h/mL) 92.6 ± 36.7 154.1 ± 61.4 a. 19 HIV-1 subjects, lopinavir and ritonavir 400/100 mg twice daily
b. 24 HIV-1 subjects, lopinavir and ritonavir 800/200 mg + emtricitabine 200 mg + tenofovir DF 300 mgSpecific PopulationsGender, Race and AgeNo gender or race related pharmacokinetic differences have been observed in adult patients. Lopinavir pharmacokinetics have not been studied in elderly patients.
Pediatric PatientsThe 230/57.5 mg/m2twice daily regimen without nevirapine and the 300/75 mg/m2twice daily regimen with nevirapine provided lopinavir plasma concentrations similar to those obtained in adult patients receiving the 400/100 mg twice daily regimen without nevirapine.
Table 15. Lopinavir Pharmacokinetic Data from Pediatric Clinical Trials, Mean ± SD Cmax(mcg/mL)Cmin(mcg/mL)AUC12(mcg•hr/m)Age ≥ 14 Days to < 6 Weeks Cohort (N = 9):5.17 ± 1.84a 1.40 ± 0.48a 43.39 ± 14.80a Age ≥ 6 Weeks to < 6 Months Cohort (N = 18):9.39 ± 4.91a 1.95 ± 1.80a 74.50 ± 37.87a Age ≥ 6 Months to ≤ 12 years Cohort (N = 24):8.2 ± 2.9b 3.4 ± 2.1b 72.6 ± 31.1b 10.0 ± 3.3c 3.6 ± 3.5c 85.8 ± 36.9c a. Lopinavir and ritonavir oral solution 300/75 mg/m2twice daily without concomitant NNRTI therapy
b. Lopinavir and ritonavir oral solution 230/57.5 mg/m2twice daily without nevirapine (n=12)
c. Lopinavir and ritonavir oral solution 300/75 mg/m2twice daily with nevirapine (n=12)PregnancyThe C12hvalues of lopinavir were lower during the second and third trimester by approximately 40% as compared to post-partum in 12 HIV-infected pregnant women received lopinavir and ritonavir 400 mg/100 mg twice daily. Yet this decrease is not considered clinically relevant in patients with no documented lopinavir and ritonavir-associated resistance substitutions receiving 400 mg/100 mg twice daily
[see Use in Specific Populations (8.1)].Renal ImpairmentLopinavir pharmacokinetics have not been studied in patients with renal impairment; however, since the renal clearance of lopinavir is negligible, a decrease in total body clearance is not expected in patients with renal impairment.
Hepatic ImpairmentMultiple dosing of lopinavir and ritonavir 400/100 mg twice daily to HIV-1 and HCV co-infected patients with mild to moderate hepatic impairment (n = 12) resulted in a 30% increase in lopinavir AUC and 20% increase in Cmaxcompared to HIV-1 infected subjects with normal hepatic function (n = 12). Additionally, the plasma protein binding of lopinavir was statistically significantly lower in both mild and moderate hepatic impairment compared to controls (99.09 vs. 99.31%, respectively). Lopinavir and ritonavir has not been studied in patients with severe hepatic impairment
[see Warnings and Precautions (5.4)andUse in Specific Populations (8.6)].Drug InteractionsLopinavir and ritonavir is an inhibitor of the P450 isoform CYP3A
in vitro. Lopinavir and ritonavir does not inhibit CYP2D6, CYP2C9, CYP2C19, CYP2E1, CYP2B6 or CYP1A2 at clinically relevant concentrations.Lopinavir and ritonavir has been shown
in vivoto induce its own metabolism and to increase the biotransformation of some drugs metabolized by cytochrome P450 enzymes and by glucuronidation.The effects of co-administration of lopinavir and ritonavir on the AUC, Cmaxand Cminare summarized in Table 16 (effect of other drugs on lopinavir) and Table 17 (effect of lopinavir and ritonavir on other drugs). For information regarding clinical recommendations, see Table 12 in Drug Interactions (7).
Table 16. Drug Interactions: Pharmacokinetic Parameters for Lopinavir in the Presence of the Co-administered Drug for Recommended Alterations in Dose or Regimen Co- administered DrugDose of Co- administered Drug(mg)Dose ofLopinavir and Ritonavir(mg)nRatio (in combination with Co-administered drug/alone) of Lopinavir Pharmacokinetic Parameters (90% CI);No Effect = 1.00CmaxAUCCminEfavirenz1 600 at bedtime 400/100 capsule twice daily 11, 73 0.97
(0.78, 1.22)0.81
(0.64, 1.03)0.61
(0.38, 0.97)600 at bedtime 500/125 tablet twice daily 19 1.12
(1.02, 1.23)1.06
(0.96, 1.17)0.90
(0.78, 1.04)600 at bedtime 600/150 tablet twice daily 23 1.36
(1.28, 1.44)1.36
(1.28, 1.44)1.32
(1.21, 1.44)Etravirine 200 twice daily 400/100 mg twice daily (tablets) 16 0.89
(0.82 to 0.96)0.87
(0.83 to 0.92)0.80
(0.73 to 0.88)Fosamprenavir2 700 twice daily plus ritonavir 100 twice daily 400/100 capsule twice daily 18 1.30
(0.85, 1.47)1.37
(0.80, 1.55)1.52
(0.72, 1.82)Ketoconazole 200 single dose 400/100 capsule twice daily 12 0.89
(0.80, 0.99)0.87
(0.75, 1.00)0.75
(0.55, 1.00)Nelfinavir 1,000 twice daily 400/100 capsule twice daily 13 0.79
(0.70, 0.89)0.73
(0.63, 0.85)0.62
(0.49, 0.78)Nevirapine 200 twice daily steady-state 400/100 capsule twice daily 22, 193 0.81
(0.62, 1.05)0.73
(0.53, 0.98)0.49
(0.28, 0.74)7 mg/kg or 4 mg/kg once daily; twice daily 1 wk5 (> 1 yr) 300/ 75 mg/m2oral solution twice daily 12, 153 0.86
(0.64, 1.16)0.78
(0.56, 1.09)0.45
(0.25, 0.81)Ombitasvir/ paritaprevir/ ritonavir+ dasabuvir2 25/150/100 +
dasabuvir 400400/100 tablet twice daily 6 0.87
(0.76, 0.99)0.94
(0.81, 1.10)1.15
(0.93, 1.42)Omeprazole 40 once daily, 5 d 400/100 tablet twice daily, 10 d 12 1.08
(0.99, 1.17)1.07
(0.99, 1.15)1.03
(0.90, 1.18)40 once daily, 5 d 800/200 tablet once daily, 10 d 12 0.94
(0.88, 1.00)0.92
(0.86, 0.99)0.71
(0.57, 0.89)Pravastatin 20 once daily, 4 d 400/100 capsule twice daily, 14 d 12 0.98
(0.89, 1.08)0.95
(0.85, 1.05)0.88
(0.77, 1.02)Ranitidine 150 single dose 400/100 tablet twice daily, 10 d 12 0.99
(0.95, 1.03)0.97
(0.93, 1.01)0.90
(0.85, 0.95)150 single dose 800/200 tablet once daily, 10 d 10 0.97
(0.95, 1.00)0.95
(0.91, 0.99)0.82
(0.74, 0.91)Rifabutin 150 once daily 400/100 capsule twice daily 14 1.08
(0.97, 1.19)1.17
(1.04, 1.31)1.20
(0.96, 1.65)Rifampin 600 once daily 400/100 capsule twice daily 22 0.45
(0.40, 0.51)0.25
(0.21, 0.29)0.01
(0.01, 0.02)600 once daily 800/200 capsule twice daily 10 1.02
(0.85, 1.23)0.84
(0.64, 1.10)0.43
(0.19, 0.96)600 once daily 400/400 capsule twice daily 9 0.93
(0.81, 1.07)0.98
(0.81, 1.17)1.03
(0.68, 1.56)Rilpivirine 150 once daily 400/100 twice daily (capsules) 15 0.96
(0.88 to 1.05)0.99
(0.89 to 1.10)0.89
(0.73 to 1.08)Ritonavir 100 twice daily 400/100 capsule twice daily 8, 213 1.28
(0.94, 1.76)1.46
(1.04, 2.06)2.16
(1.29, 3.62)Tipranavir/ ritonavir 500/200 twice daily 400/100 capsule twice daily 21, 693 0.53
(0.40, 0.69)0.45
(0.32, 0.63)0.30
(0.17, 0.51)
0.484
(0.40, 0.58)1.000000000000000e+00Reference for comparison is lopinavir/ritonavir 400/100 mg twice daily without efavirenz.
2.000000000000000e+00Data extracted from the U.S. prescribing information of co-administered drugs.
3.000000000000000e+00Parallel group design
4.000000000000000e+00Drug levels obtained at 8 to 16 hours post dose
N/A = Not available.Table 17. Drug Interactions: Pharmacokinetic Parameters for Co-administered Drug in the Presence of Lopinavir and Ritonavir for Recommended Alterations in Dose or Regimen Co- administered DrugDose of Co- administered Drug (mg)Dose ofLopinavir and Ritonavir(mg)nRatio (in combination withLopinavir and Ritonavir/alone) of Co-administered Drug Pharmacokinetic Parameters (90% CI); No Effect = 1.00CmaxAUCCminBedaquiline1 400 single dose 400/100 twice daily N/A N/A 1.22
(1.11, 1.34)N/A Efavirenz 600 at bedtime 400/100
capsule twice daily11, 123 0.91
(0.72, 1.15)0.84
(0.62, 1.15)0.84
(0.58, 1.20)Elbasvir/ grazoprevir1 50 once daily 400/100 twice daily 10 2.87
(2.29, 3.58)3.71
(3.05, 4.53)4.58
(3.72, 5.64)200 once daily 13 7.31
(5.65, 9.45)12.86
(10.25, 16.13)21.70
(12.99, 36.25)Ethinyl Estradiol 35 mcg once daily
(Ortho Novum®)400/100
capsule twice daily12 0.59
(0.52, 0.66)0.58
(0.54, 0.62)0.42
(0.36, 0.49)Etravirine 200 twice daily 400/100 tablet twice day 16 0.70
(0.64 to 0.78)0.65
(0.59 to 0.71)0.55
(0.49 to 0.62)Fosamprenavir1 700 twice daily plus ritonavir 100 twice daily 400/100
capsule twice daily18 0.42
(0.30, 0.58)0.37
(0.28, 0.49)0.35
(0.27, 0.46)Indinavir 600 twice daily combo nonfasting vs. 800 three times daily alone fasting 400/100
capsule twice daily13 0.71
(0.63, 0.81)0.91
(0.75, 1.10)3.47
(2.60, 4.64)Ketoconazole 200 single dose 400/100
capsule twice daily12 1.13
(0.91, 1.40)3.04
(2.44, 3.79)N/A Maraviroc1 300 twice daily 400/100
twice daily11 1.97
(1.66, 2.34)3.95
(3.43, 4.56)9.24
(7.98, 10.7)Methadone 5 single dose 400/100
capsule twice daily11 0.55
(0.48, 0.64)0.47
(0.42, 0.53)N/A Nelfinavir 1,000 twice daily combo vs.
1,250 twice daily alone400/100
capsule twice daily13 0.93
(0.82, 1.05)1.07
(0.95, 1.19)1.86
(1.57, 2.22)M8 metabolite 2.36
(1.91, 2.91)3.46
(2.78, 4.31)7.49
(5.85, 9.58)Nevirapine 200 once daily twice daily 400/100
capsule twice daily5, 63 1.05
(0.72, 1.52)1.08
(0.72, 1.64)1.15
(0.71, 1.86)Norethindrone 1 once daily
(Ortho Novum®)400/100
capsule twice daily12 0.84
(0.75, 0.94)0.83
(0.73, 0.94)0.68
(0.54, 0.85)Ombitasvir/ paritaprevir/ ritonavir+ dasabuvir1 25/150/100 +
dasabuvir 400400/100
tablet twice
daily6 1.14
(1.01, 1.28)1.17
(1.07, 1.28)1.24
(1.14, 1.34)2.04
(1.30, 3.20)2.17
(1.63, 2.89)2.36
(1.00, 5.55)1.55
(1.16, 2.09)2.05
(1.49, 2.81)5.25
(3.33, 8.28)0.99
(0.75, 1.31)0.93
(0.75, 1.15)0.68
(0.57, 0.80)Pitavastatin1 4 once daily 400/100 tablet twice daily 23 0.96
(0.84 to 1.10)0.80
(0.73 to 0.87)N/A Pravastatin 20 once daily 400/100 capsule twice daily 12 1.26
(0.87, 1.83)1.33
(0.91, 1.94)N/A Rifabutin 150 once daily
combo vs. 300
once daily
alone400/100 capsule twice daily 12 2.12
(1.89, 2.38)3.03
(2.79, 3.30)4.90
(3.18, 5.76)25- O-desacetyl rifabutin23.6
(13.7, 25.3)47.5
(29.3, 51.8)94.9
(74.0, 122)Rifabutin + 25- O-desacetyl rifabutin3.46
(3.07, 3.91)5.73
(5.08, 6.46)9.53
(7.56, 12.01)Rilpivirine 150 once daily 400/100 capsules twice daily 15 1.29
(1.18 to 1.40)1.52
(1.36 to 1.70)1.74
(1.46 to 2.08)Rosuvastatin2 20 once daily 400/100 tablet twice daily 15 4.66
(3.4, 6.4)2.08
(1.66, 2.6)1.04
(0.9, 1.2)Tenofovir alafenamide1 10 once daily 800/200
tablet once daily10 2.19
(1.72, 2.79)1.47
(1.17, 1.85)N/A Tenofovir disoproxil fumarate1 300 once daily 400/100 capsule twice daily 24 No Change 1.32
(1.26, 1.38)1.51
(1.32, 1.66)1.000000000000000e+00Data extracted from the U.S. prescribing information of co-administered drugs.
2.000000000000000e+00Kiser, et al. J Acquir Immune Defic Syndr. 2008 Apr 15; 47(5):570-8.
3Parallel group design
N/A = Not available. - In pregnant women. (,
2.5 Dosage Recommendations in PregnancyAdminister 400/100 mg of lopinavir and ritonavir twice daily in pregnant patients with no documented lopinavir-associated resistance substitutions.
- Once daily lopinavir and ritonavir dosing is not recommended in pregnancy[ see Use in Specific Populations (8.1)and Clinical Pharmacology (12.3)].
- There are insufficient data to recommend dosing in pregnant women with any documented lopinavir-associated resistance substitutions.
- No dosage adjustment of lopinavir and ritonavir is required for patients during the postpartum period.
- Avoid use of lopinavir and ritonavir oral solution in pregnant women[see Use in Specific Populations (8.1)].
,8.1 PregnancyPregnancy Exposure RegistryThere is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to lopinavir and ritonavir during pregnancy. Physicians are encouraged to register patients by calling the Antiretroviral Pregnancy Registry at 1-800-258-4263.
Risk SummaryAvailable data from the Antiretroviral Pregnancy Registry show no difference in the risk of overall major birth defects compared to the background rate for major birth defects of 2.7% in the U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP)
(see Data). The estimated background rate of miscarriage in clinically recognized pregnancies in the U.S. general population is 15 to 20%. The background risk for major birth defects and miscarriage for the indicated population is unknown. Methodological limitations of the APR include the use of MACDP as the external comparator group. The MACDP population is not disease-specific, evaluates women and infants from a limited geographic area, and does not include outcomes for births that occurred at <20 weeks gestation(see Data). No treatment- related malformations were observed when lopinavir in combination with ritonavir was administered to pregnant rats or rabbits; however embryonic and fetal developmental toxicities occurred in rats administered maternally toxic doses.Clinical ConsiderationsDose Adjustments During Pregnancy and the Postpartum PeriodAdminister 400/100 mg of lopinavir and ritonavir twice daily in pregnant patients with no documented lopinavir-associated resistance substitutions
[see Dosage and Administration (2.5)and Clinical Pharmacology (12.3)]. There are insufficient data to recommend lopinavir and ritonavir dosing for pregnant patients with any documented lopinavir-associated resistance substitutions. No dose adjustment of lopinavir and ritonavir is required for patients during the postpartum period.Once daily lopinavir and ritonavir dosing is not recommended in pregnancy.
Avoid use of lopinavir and ritonavir oral solution during pregnancy due to the ethanol content. lopinavir and ritonavir oral solution contains the excipients ethanol, approximately 42% (v/v and propylene glycol, approximately 15%.
DataHuman DataLopinavir and ritonavir was evaluated in 12 HIV-infected pregnant women in an open-label pharmacokinetic trial
[see Clinical Pharmacology (12.3)]. No new trends in the safety profile were identified in pregnant women dosed with lopinavir and ritonavir compared to the safety described in non-pregnant adults, based on the review of these limited data.Antiretroviral Pregnancy Registry Data: Based on prospective reports from the Antiretroviral Pregnancy Registry (APR) of over 3,000 exposures to lopinavir containing regimens (including over 1,000 exposed in the first trimester), there was no difference between lopinavir and overall birth defects compared with the background birth defect rate of 2.7% in the U.S. reference population of the Metropolitan Atlanta Congenital Defects Program. The prevalence of birth defects in live births was 2.1% (95% CI: 1.4% to 3%) following first-trimester exposure to lopinavir-containing regimens and 3% (95% CI: 2.4% to 3.8%) following second and third trimester exposure to lopinavir-containing regimens. Based on prospective reports from the APR of over 5,000 exposures to ritonavir containing regimens (including over 2,000 exposures in the first trimester) there was no difference between ritonavir and overall birth defects compared with the U.S. background rate (MACDP). The prevalence of birth defects in live births was 2.2% (95% CI: 1.7% to 2.8%) following first-trimester exposure to ritonavir-containing regimens and 2.9% (95% CI: 2.4% to 3.6%) following second and third trimester exposure to ritonavir- containing regimens. For both lopinavir and ritonavir, sufficient numbers of first trimester exposures have been monitored to detect at least a 1.5 fold increase in risk of overall birth defects and a 2 fold increase in risk of birth defects in the cardiovascular and genitourinary systems.
Animal DataEmbryonic and fetal developmental toxicities (early resorption, decreased fetal viability, decreased fetal body weight, increased incidence of skeletal variations and skeletal ossification delays) occurred in rats administered lopinavir in combination with ritonavir (on gestation days 6 to 17) at a maternally toxic dosage. Based on AUC measurements, the drug exposures in rats at the toxic doses were approximately 0.7 times (for lopinavir) and 1.8 times (for ritonavir) the exposures in humans at the recommended therapeutic dose (400/100 mg twice daily). In a pre- and post-natal study in rats, a developmental toxicity (a decrease in survival in pups between birth and postnatal Day 21) occurred.
No embryonic and fetal developmental toxicities were observed in rabbits administered lopinavir in combination with ritonavir (on gestation days 6 to 18) at a maternally toxic dosage. Based on AUC measurements, the drug exposures in rabbits at the toxic doses were approximately 0.6 times (for lopinavir) and similar to (for ritonavir) the exposures in humans at the recommended therapeutic dose (400/100 mg twice daily).
)12.3 PharmacokineticsThe pharmacokinetic properties of lopinavir are summarized in Table 13. The steady-state pharmacokinetic parameters of lopinavir are summarized in Table 14. Under fed conditions, lopinavir concentrations were similar following administration of lopinavir and ritonavir tablets to capsules with less pharmacokinetic variability. Under fed conditions (500 kcal, 25% from fat), lopinavir concentrations were similar following administration of lopinavir and ritonavir capsules and oral solution.
Table 13. Pharmacokinetic Properties of Lopinavir AbsorptionTmax(hr)a 4.4 ± 0.8 Effect of meal
(relative to fasting)
Tablet
Oral solution
↑ 19%b
↑ 130%bDistribution% Bound to human plasma proteins > 98 Vd/Fa(L) 16.9 MetabolismMetabolism CYP3A EliminationMajor route of elimination hepatic t1/2(h)a 6.9 ± 2.2 % of dose excreted in urine 10.4 ± 2.3 % of dose excreted in feces 82.6 ± 2.5 a.Lopinavir and ritonavir tablet
b.Changes in AUC valuesTable 14. Steady-State Pharmacokinetic Parameters of Lopinavir, Mean ± SD Pharmacokinetic ParameterTwice DailyaOnce DailybCmax(mcg/mL) 9.8 ± 3.7 11.8 ± 3.7 Cmin(mcg/mL) 5.5 ± 2.7 1.7 ± 1.6 AUCtau(mcg•h/mL) 92.6 ± 36.7 154.1 ± 61.4 a. 19 HIV-1 subjects, lopinavir and ritonavir 400/100 mg twice daily
b. 24 HIV-1 subjects, lopinavir and ritonavir 800/200 mg + emtricitabine 200 mg + tenofovir DF 300 mgSpecific PopulationsGender, Race and AgeNo gender or race related pharmacokinetic differences have been observed in adult patients. Lopinavir pharmacokinetics have not been studied in elderly patients.
Pediatric PatientsThe 230/57.5 mg/m2twice daily regimen without nevirapine and the 300/75 mg/m2twice daily regimen with nevirapine provided lopinavir plasma concentrations similar to those obtained in adult patients receiving the 400/100 mg twice daily regimen without nevirapine.
Table 15. Lopinavir Pharmacokinetic Data from Pediatric Clinical Trials, Mean ± SD Cmax(mcg/mL)Cmin(mcg/mL)AUC12(mcg•hr/m)Age ≥ 14 Days to < 6 Weeks Cohort (N = 9):5.17 ± 1.84a 1.40 ± 0.48a 43.39 ± 14.80a Age ≥ 6 Weeks to < 6 Months Cohort (N = 18):9.39 ± 4.91a 1.95 ± 1.80a 74.50 ± 37.87a Age ≥ 6 Months to ≤ 12 years Cohort (N = 24):8.2 ± 2.9b 3.4 ± 2.1b 72.6 ± 31.1b 10.0 ± 3.3c 3.6 ± 3.5c 85.8 ± 36.9c a. Lopinavir and ritonavir oral solution 300/75 mg/m2twice daily without concomitant NNRTI therapy
b. Lopinavir and ritonavir oral solution 230/57.5 mg/m2twice daily without nevirapine (n=12)
c. Lopinavir and ritonavir oral solution 300/75 mg/m2twice daily with nevirapine (n=12)PregnancyThe C12hvalues of lopinavir were lower during the second and third trimester by approximately 40% as compared to post-partum in 12 HIV-infected pregnant women received lopinavir and ritonavir 400 mg/100 mg twice daily. Yet this decrease is not considered clinically relevant in patients with no documented lopinavir and ritonavir-associated resistance substitutions receiving 400 mg/100 mg twice daily
[see Use in Specific Populations (8.1)].Renal ImpairmentLopinavir pharmacokinetics have not been studied in patients with renal impairment; however, since the renal clearance of lopinavir is negligible, a decrease in total body clearance is not expected in patients with renal impairment.
Hepatic ImpairmentMultiple dosing of lopinavir and ritonavir 400/100 mg twice daily to HIV-1 and HCV co-infected patients with mild to moderate hepatic impairment (n = 12) resulted in a 30% increase in lopinavir AUC and 20% increase in Cmaxcompared to HIV-1 infected subjects with normal hepatic function (n = 12). Additionally, the plasma protein binding of lopinavir was statistically significantly lower in both mild and moderate hepatic impairment compared to controls (99.09 vs. 99.31%, respectively). Lopinavir and ritonavir has not been studied in patients with severe hepatic impairment
[see Warnings and Precautions (5.4)andUse in Specific Populations (8.6)].Drug InteractionsLopinavir and ritonavir is an inhibitor of the P450 isoform CYP3A
in vitro. Lopinavir and ritonavir does not inhibit CYP2D6, CYP2C9, CYP2C19, CYP2E1, CYP2B6 or CYP1A2 at clinically relevant concentrations.Lopinavir and ritonavir has been shown
in vivoto induce its own metabolism and to increase the biotransformation of some drugs metabolized by cytochrome P450 enzymes and by glucuronidation.The effects of co-administration of lopinavir and ritonavir on the AUC, Cmaxand Cminare summarized in Table 16 (effect of other drugs on lopinavir) and Table 17 (effect of lopinavir and ritonavir on other drugs). For information regarding clinical recommendations, see Table 12 in Drug Interactions (7).
Table 16. Drug Interactions: Pharmacokinetic Parameters for Lopinavir in the Presence of the Co-administered Drug for Recommended Alterations in Dose or Regimen Co- administered DrugDose of Co- administered Drug(mg)Dose ofLopinavir and Ritonavir(mg)nRatio (in combination with Co-administered drug/alone) of Lopinavir Pharmacokinetic Parameters (90% CI);No Effect = 1.00CmaxAUCCminEfavirenz1 600 at bedtime 400/100 capsule twice daily 11, 73 0.97
(0.78, 1.22)0.81
(0.64, 1.03)0.61
(0.38, 0.97)600 at bedtime 500/125 tablet twice daily 19 1.12
(1.02, 1.23)1.06
(0.96, 1.17)0.90
(0.78, 1.04)600 at bedtime 600/150 tablet twice daily 23 1.36
(1.28, 1.44)1.36
(1.28, 1.44)1.32
(1.21, 1.44)Etravirine 200 twice daily 400/100 mg twice daily (tablets) 16 0.89
(0.82 to 0.96)0.87
(0.83 to 0.92)0.80
(0.73 to 0.88)Fosamprenavir2 700 twice daily plus ritonavir 100 twice daily 400/100 capsule twice daily 18 1.30
(0.85, 1.47)1.37
(0.80, 1.55)1.52
(0.72, 1.82)Ketoconazole 200 single dose 400/100 capsule twice daily 12 0.89
(0.80, 0.99)0.87
(0.75, 1.00)0.75
(0.55, 1.00)Nelfinavir 1,000 twice daily 400/100 capsule twice daily 13 0.79
(0.70, 0.89)0.73
(0.63, 0.85)0.62
(0.49, 0.78)Nevirapine 200 twice daily steady-state 400/100 capsule twice daily 22, 193 0.81
(0.62, 1.05)0.73
(0.53, 0.98)0.49
(0.28, 0.74)7 mg/kg or 4 mg/kg once daily; twice daily 1 wk5 (> 1 yr) 300/ 75 mg/m2oral solution twice daily 12, 153 0.86
(0.64, 1.16)0.78
(0.56, 1.09)0.45
(0.25, 0.81)Ombitasvir/ paritaprevir/ ritonavir+ dasabuvir2 25/150/100 +
dasabuvir 400400/100 tablet twice daily 6 0.87
(0.76, 0.99)0.94
(0.81, 1.10)1.15
(0.93, 1.42)Omeprazole 40 once daily, 5 d 400/100 tablet twice daily, 10 d 12 1.08
(0.99, 1.17)1.07
(0.99, 1.15)1.03
(0.90, 1.18)40 once daily, 5 d 800/200 tablet once daily, 10 d 12 0.94
(0.88, 1.00)0.92
(0.86, 0.99)0.71
(0.57, 0.89)Pravastatin 20 once daily, 4 d 400/100 capsule twice daily, 14 d 12 0.98
(0.89, 1.08)0.95
(0.85, 1.05)0.88
(0.77, 1.02)Ranitidine 150 single dose 400/100 tablet twice daily, 10 d 12 0.99
(0.95, 1.03)0.97
(0.93, 1.01)0.90
(0.85, 0.95)150 single dose 800/200 tablet once daily, 10 d 10 0.97
(0.95, 1.00)0.95
(0.91, 0.99)0.82
(0.74, 0.91)Rifabutin 150 once daily 400/100 capsule twice daily 14 1.08
(0.97, 1.19)1.17
(1.04, 1.31)1.20
(0.96, 1.65)Rifampin 600 once daily 400/100 capsule twice daily 22 0.45
(0.40, 0.51)0.25
(0.21, 0.29)0.01
(0.01, 0.02)600 once daily 800/200 capsule twice daily 10 1.02
(0.85, 1.23)0.84
(0.64, 1.10)0.43
(0.19, 0.96)600 once daily 400/400 capsule twice daily 9 0.93
(0.81, 1.07)0.98
(0.81, 1.17)1.03
(0.68, 1.56)Rilpivirine 150 once daily 400/100 twice daily (capsules) 15 0.96
(0.88 to 1.05)0.99
(0.89 to 1.10)0.89
(0.73 to 1.08)Ritonavir 100 twice daily 400/100 capsule twice daily 8, 213 1.28
(0.94, 1.76)1.46
(1.04, 2.06)2.16
(1.29, 3.62)Tipranavir/ ritonavir 500/200 twice daily 400/100 capsule twice daily 21, 693 0.53
(0.40, 0.69)0.45
(0.32, 0.63)0.30
(0.17, 0.51)
0.484
(0.40, 0.58)1.000000000000000e+00Reference for comparison is lopinavir/ritonavir 400/100 mg twice daily without efavirenz.
2.000000000000000e+00Data extracted from the U.S. prescribing information of co-administered drugs.
3.000000000000000e+00Parallel group design
4.000000000000000e+00Drug levels obtained at 8 to 16 hours post dose
N/A = Not available.Table 17. Drug Interactions: Pharmacokinetic Parameters for Co-administered Drug in the Presence of Lopinavir and Ritonavir for Recommended Alterations in Dose or Regimen Co- administered DrugDose of Co- administered Drug (mg)Dose ofLopinavir and Ritonavir(mg)nRatio (in combination withLopinavir and Ritonavir/alone) of Co-administered Drug Pharmacokinetic Parameters (90% CI); No Effect = 1.00CmaxAUCCminBedaquiline1 400 single dose 400/100 twice daily N/A N/A 1.22
(1.11, 1.34)N/A Efavirenz 600 at bedtime 400/100
capsule twice daily11, 123 0.91
(0.72, 1.15)0.84
(0.62, 1.15)0.84
(0.58, 1.20)Elbasvir/ grazoprevir1 50 once daily 400/100 twice daily 10 2.87
(2.29, 3.58)3.71
(3.05, 4.53)4.58
(3.72, 5.64)200 once daily 13 7.31
(5.65, 9.45)12.86
(10.25, 16.13)21.70
(12.99, 36.25)Ethinyl Estradiol 35 mcg once daily
(Ortho Novum®)400/100
capsule twice daily12 0.59
(0.52, 0.66)0.58
(0.54, 0.62)0.42
(0.36, 0.49)Etravirine 200 twice daily 400/100 tablet twice day 16 0.70
(0.64 to 0.78)0.65
(0.59 to 0.71)0.55
(0.49 to 0.62)Fosamprenavir1 700 twice daily plus ritonavir 100 twice daily 400/100
capsule twice daily18 0.42
(0.30, 0.58)0.37
(0.28, 0.49)0.35
(0.27, 0.46)Indinavir 600 twice daily combo nonfasting vs. 800 three times daily alone fasting 400/100
capsule twice daily13 0.71
(0.63, 0.81)0.91
(0.75, 1.10)3.47
(2.60, 4.64)Ketoconazole 200 single dose 400/100
capsule twice daily12 1.13
(0.91, 1.40)3.04
(2.44, 3.79)N/A Maraviroc1 300 twice daily 400/100
twice daily11 1.97
(1.66, 2.34)3.95
(3.43, 4.56)9.24
(7.98, 10.7)Methadone 5 single dose 400/100
capsule twice daily11 0.55
(0.48, 0.64)0.47
(0.42, 0.53)N/A Nelfinavir 1,000 twice daily combo vs.
1,250 twice daily alone400/100
capsule twice daily13 0.93
(0.82, 1.05)1.07
(0.95, 1.19)1.86
(1.57, 2.22)M8 metabolite 2.36
(1.91, 2.91)3.46
(2.78, 4.31)7.49
(5.85, 9.58)Nevirapine 200 once daily twice daily 400/100
capsule twice daily5, 63 1.05
(0.72, 1.52)1.08
(0.72, 1.64)1.15
(0.71, 1.86)Norethindrone 1 once daily
(Ortho Novum®)400/100
capsule twice daily12 0.84
(0.75, 0.94)0.83
(0.73, 0.94)0.68
(0.54, 0.85)Ombitasvir/ paritaprevir/ ritonavir+ dasabuvir1 25/150/100 +
dasabuvir 400400/100
tablet twice
daily6 1.14
(1.01, 1.28)1.17
(1.07, 1.28)1.24
(1.14, 1.34)2.04
(1.30, 3.20)2.17
(1.63, 2.89)2.36
(1.00, 5.55)1.55
(1.16, 2.09)2.05
(1.49, 2.81)5.25
(3.33, 8.28)0.99
(0.75, 1.31)0.93
(0.75, 1.15)0.68
(0.57, 0.80)Pitavastatin1 4 once daily 400/100 tablet twice daily 23 0.96
(0.84 to 1.10)0.80
(0.73 to 0.87)N/A Pravastatin 20 once daily 400/100 capsule twice daily 12 1.26
(0.87, 1.83)1.33
(0.91, 1.94)N/A Rifabutin 150 once daily
combo vs. 300
once daily
alone400/100 capsule twice daily 12 2.12
(1.89, 2.38)3.03
(2.79, 3.30)4.90
(3.18, 5.76)25- O-desacetyl rifabutin23.6
(13.7, 25.3)47.5
(29.3, 51.8)94.9
(74.0, 122)Rifabutin + 25- O-desacetyl rifabutin3.46
(3.07, 3.91)5.73
(5.08, 6.46)9.53
(7.56, 12.01)Rilpivirine 150 once daily 400/100 capsules twice daily 15 1.29
(1.18 to 1.40)1.52
(1.36 to 1.70)1.74
(1.46 to 2.08)Rosuvastatin2 20 once daily 400/100 tablet twice daily 15 4.66
(3.4, 6.4)2.08
(1.66, 2.6)1.04
(0.9, 1.2)Tenofovir alafenamide1 10 once daily 800/200
tablet once daily10 2.19
(1.72, 2.79)1.47
(1.17, 1.85)N/A Tenofovir disoproxil fumarate1 300 once daily 400/100 capsule twice daily 24 No Change 1.32
(1.26, 1.38)1.51
(1.32, 1.66)1.000000000000000e+00Data extracted from the U.S. prescribing information of co-administered drugs.
2.000000000000000e+00Kiser, et al. J Acquir Immune Defic Syndr. 2008 Apr 15; 47(5):570-8.
3Parallel group design
N/A = Not available. - Once daily lopinavir and ritonavir dosing is not recommended in pregnancy
- Adult patients with three or more of the following lopinavir resistance-associated substitutions: L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V, I54L/T/V, V82A/C/F/S/T, and I84V. (
Lopinavir and ritonavir tablets and oral solution are not recommended for once daily dosing in pediatric patients younger than 18 years of age. The dose of the oral solution should be administered using the calibrated cup (supplied) or oral dosing syringe. Lopinavir and ritonavir 100/25 mg tablets should be considered only in children who have reliably demonstrated the ability to swallow the intact tablet.
Lopinavir and ritonavir oral solution is not recommended in neonates before a postmenstrual age (first day of the mother’s last menstrual period to birth plus the time elapsed after birth) of 42 weeks and a postnatal age of at least 14 days has been attained
Lopinavir and ritonavir oral solution contains approximately 42% (v/v) ethanol and approximately 15% (w/v) propylene glycol. Total amounts of ethanol and propylene glycol from all medicines that are to be given to pediatric patients 14 days to 6 months of age should be taken into account in order to avoid toxicity from these excipients
Calculate the appropriate dose of lopinavir and ritonavir for each individual pediatric patient based on body weight (kg) or body surface area (BSA) to avoid underdosing or exceeding the recommended adult dose.
Body surface area (BSA) can be calculated as follows: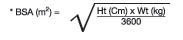
The lopinavir and ritonavir dose can be calculated based on weight or BSA:
Patient Weight (kg) × Prescribed lopinavir dose (mg/kg) = Administered lopinavir dose (mg)
Patient BSA (m2) × Prescribed lopinavir dose (mg/m2) = Administered lopinavir dose (mg)
If lopinavir and ritonavir oral solution is used, the volume (mL) of lopinavir and ritonavir solution can be determined as follows:
Volume of lopinavir and ritonavir solution (mL) = Administered lopinavir dose (mg) ÷ 80 (mg/mL)
Table 4 summarizes the recommended daily dosing regimen for pediatric patients 14 days to less than 18 years of age using the oral solution.
Lopinavir and ritonavir administered in combination with efavirenz, nevirapine, or nelfinavir in patients younger than 6 months of age is not recommended. Total dose of lopinavir and ritonavir oral solution in pediatric patients should not exceed the recommended adult daily dose of 400/100 mg (5mL) twice daily.
Patient Age | Based on Weight (mg/kg) | Based on BSA (mg/m2) | Frequency | |
| 14 days to 6 months | 16/4 | 300/75 | Given twice daily | |
| Older than 6 months to less than 18 years | Less than 15 kg | 12/3 | 230/57.5 | Given twice daily |
| 15 kg to 40 kg | 10/2.5 | |||
Table 5 provides the dosing recommendations for pediatric patients older than 6 months to less than 18 years of age based on body weight or body surface area for lopinavir and ritonavir tablets.
Body Weight (kg) | Body Surface Area (m2)* | Recommended number of 100/25 mg Tablets Twice Daily |
| ≥15 to 25 | ≥0.6 to < 0.9 | 2 |
| >25 to 35 | ≥0.9 to < 1.4 | 3 |
| >35 | ≥1.4 | 4 |
| * Lopinavir and ritonavir oral solution is available for children with a BSA less than 0.6 m2or those who are unable to reliably swallow a tablet. | ||
Table 6 provides the dosing recommendations for pediatric patients older than 6 months to less than 18 years of age based on body weight or body surface area for Lopinavir and Ritonavir Oral Solution when given in combination with efavirenz, nevirapine, or nelfinavir:
Patient Age | Based on Weight (mg/kg) | Based on BSA (mg/m2) | Frequency | |
| > 6 months to < 18 years | <15 kg | 13/3.25 | 300/75 | Given twice daily |
| ≥15 kg to 45 kg | 11/2.75 | |||
Table 7 provides the dosing recommendations for pediatric patients older than 6 months to less than 18 years of age based on body weight or body surface area for lopinavir and ritonavir tablets when given in combination with efavirenz, nevirapine, or nelfinavir.
Body Weight (kg) | Body Surface Area (m2)* | Recommended number of 100/25 mg Tablets Twice Daily |
| ≥15 to 20 | ≥0.6 to < 0.8 | 2 |
| >20 to 30 | ≥0.8 to < 1.2 | 3 |
| >30 to 45 | ≥1.2 to <1.7 | 4 |
| >45 | ≥1.7 | 5 [see Dosage and Administration (2.4)] |
| * Lopinavir and ritonavir oral solution is available for children with a BSA less than 0.6 m2or those who are unable to reliably swallow a tablet. † Please refer to the individual product labels for appropriate dosing in children. | ||
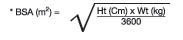
- Lopinavir and ritonavir once daily dosing regimen is not recommended in pediatric patients.
- Twice daily dose is based on body weight or body surface area.
- Dose adjustments of lopinavir and ritonavir may be needed when co-administering with efavirenz, nevirapine, or nelfinavir. (,
2.3 Dosage Recommendations in AdultsLopinavir and ritonavir can be given in once daily or twice daily dosing regimen at dosages noted in Tables 1 and 2. Lopinavir and ritonavir once daily dosing regimen is not recommended in:- Adult patients with three or more of the following lopinavir resistance-associated substitutions: L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V, I54L/T/V, V82A/C/F/S/T, and I84V[ see Microbiology (12.4)].
- In combination with carbamazepine, phenobarbital, or phenytoin[ see Drug Interactions (7.3)].
- In combination with efavirenz, nevirapine, or nelfinavir[ see Drug Interactions (7.3)and Clinical Pharmacology (12.3)].
- In pediatric patients younger than 18 years of age[ see Dosage and Administration (2.4)].
- In pregnant women[ see Dosage and Administration (2.5), Use in Specific Populations (8.1)and Clinical Pharmacology (12.3)].
Table 1. Recommended Dosage in Adults - Lopinavir and Ritonavir Once Daily Regimen Lopinavir and Ritonavir Dosage FormRecommended Dosage200 mg/50 mg Tablets 800 mg/200 mg (4 tablets) once daily 80 mg/20 mg per mL Oral Solution 800 mg/200 mg (10 mL) once daily Table 2. Recommended Dosage in Adults - Lopinavir and Ritonavir Twice Daily Regimen Lopinavir and Ritonavir Dosage FormRecommended Dosage200 mg/50 mg Tablets 400 mg/100 mg (2 tablets) twice daily 80 mg/20 mg per mL Oral Solution 400 mg/100 mg (5 mL) twice daily The dose of lopinavir and ritonavir must be increased when administered in combination with efavirenz, nevirapine or nelfinavir. Table 3 outlines the dosage recommendations for twice daily dosing when lopinavir and ritonavir is taken in combination with these agents.
Table 3. Recommended Dosage in Adults - Lopinavir and Ritonavir Twice Daily Regimen in Combination with Efavirenz, Nevirapine, or Nelfinavir Lopinavir and Ritonavir Dosage FormRecommended Dosage200 mg/50 mg Tablets and 100 mg/25 mg Tablets 500 mg/125 mg (2 tablets of 200 mg/50 mg
+ 1 tablet of 100 mg/25 mg) twice daily80 mg/20 mg per mL Oral Solution 520 mg/130 mg (6.5 mL) twice daily ,2.4 Dosage Recommendations in Pediatric PatientsLopinavir and ritonavir tablets and oral solution are not recommended for once daily dosing in pediatric patients younger than 18 years of age. The dose of the oral solution should be administered using the calibrated cup (supplied) or oral dosing syringe. Lopinavir and ritonavir 100/25 mg tablets should be considered only in children who have reliably demonstrated the ability to swallow the intact tablet.
Lopinavir and ritonavir oral solution is not recommended in neonates before a postmenstrual age (first day of the mother’s last menstrual period to birth plus the time elapsed after birth) of 42 weeks and a postnatal age of at least 14 days has been attained
[seeWarnings and Precautions (5.2)].Lopinavir and ritonavir oral solution contains approximately 42% (v/v) ethanol and approximately 15% (w/v) propylene glycol. Total amounts of ethanol and propylene glycol from all medicines that are to be given to pediatric patients 14 days to 6 months of age should be taken into account in order to avoid toxicity from these excipients
[see Warnings and Precautions (5.2)andOverdosage (10)].Pediatric Dosage CalculationsCalculate the appropriate dose of lopinavir and ritonavir for each individual pediatric patient based on body weight (kg) or body surface area (BSA) to avoid underdosing or exceeding the recommended adult dose.
Body surface area (BSA) can be calculated as follows:
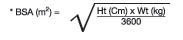
The lopinavir and ritonavir dose can be calculated based on weight or BSA:
Based on Weight:Patient Weight (kg) × Prescribed lopinavir dose (mg/kg) = Administered lopinavir dose (mg)
Based on BSA:Patient BSA (m2) × Prescribed lopinavir dose (mg/m2) = Administered lopinavir dose (mg)
If lopinavir and ritonavir oral solution is used, the volume (mL) of lopinavir and ritonavir solution can be determined as follows:
Volume of lopinavir and ritonavir solution (mL) = Administered lopinavir dose (mg) ÷ 80 (mg/mL)
Oral Solution Dosage Recommendation in Pediatric Patients 14 Days to Less Than 18 Years:Table 4 summarizes the recommended daily dosing regimen for pediatric patients 14 days to less than 18 years of age using the oral solution.
Lopinavir and ritonavir administered in combination with efavirenz, nevirapine, or nelfinavir in patients younger than 6 months of age is not recommended. Total dose of lopinavir and ritonavir oral solution in pediatric patients should not exceed the recommended adult daily dose of 400/100 mg (5mL) twice daily.
Table 4. Lopinavir and Ritonavir Oral Solution Daily Dosage Recommendations in Pediatric Patients 14 days to Less Than 18 Years Without Concomitant Efavirenz, Nevirapine, or Nelfinavir Patient AgeBased on Weight(mg/kg)Based on BSA(mg/m2)Frequency14 days to 6 months 16/4 300/75 Given twice
dailyOlder than 6 months to less than 18 years Less than 15 kg 12/3 230/57.5 Given twice
daily15 kg to 40 kg 10/2.5 Tablet Dosage Recommendation in Pediatric Patients Older than 6 Months to Less than 18 Years:Table 5 provides the dosing recommendations for pediatric patients older than 6 months to less than 18 years of age based on body weight or body surface area for lopinavir and ritonavir tablets.
Table 5. Lopinavir and Ritonavir Tablet Daily Dosage Recommendations in Pediatric Patients > 6 Months to < 18 Years of Age Without Concomitant Efavirenz, Nevirapine, or Nelfinavir Body Weight (kg)Body Surface Area (m2)*Recommended numberof 100/25 mg TabletsTwice Daily≥15 to 25 ≥0.6 to < 0.9 2 >25 to 35 ≥0.9 to < 1.4 3 >35 ≥1.4 4 * Lopinavir and ritonavir oral solution is available for children with a BSA less than 0.6 m2or those who are unable to reliably swallow a tablet. Concomitant Therapy: Efavirenz, Nevirapine, or NelfinavirDosing recommendations using oral solutionTable 6 provides the dosing recommendations for pediatric patients older than 6 months to less than 18 years of age based on body weight or body surface area for Lopinavir and Ritonavir Oral Solution when given in combination with efavirenz, nevirapine, or nelfinavir:
Table 6. Lopinavir and Ritonavir Oral Solution Daily Dosage Recommendations for Pediatric Patients >6 Months to < 18 Years of Age With Concomitant Efavirenz, Nevirapine, or Nelfinavir Patient AgeBased on Weight(mg/kg)Based on BSA(mg/m2)Frequency> 6 months to
< 18 years<15 kg 13/3.25 300/75 Given twice daily ≥15 kg to 45 kg 11/2.75 Dosing recommendations using tabletsTable 7 provides the dosing recommendations for pediatric patients older than 6 months to less than 18 years of age based on body weight or body surface area for lopinavir and ritonavir tablets when given in combination with efavirenz, nevirapine, or nelfinavir.
Table 7. Lopinavir and Ritonavir Tablet Daily Dosage Recommendations for Pediatric Patients > 6 Months to < 18 Years of Age With Concomitant Efavirenz†, Nevirapine, or Nelfinavir† Body Weight (kg)Body Surface Area (m2)*Recommended number of 100/25 mg Tablets Twice Daily≥15 to 20 ≥0.6 to < 0.8 2 >20 to 30 ≥0.8 to < 1.2 3 >30 to 45 ≥1.2 to <1.7 4 >45 ≥1.7 5 [see Dosage and Administration (2.4)]* Lopinavir and ritonavir oral solution is available for children with a BSA less than 0.6 m2or those who are unable to reliably swallow a tablet. †Please refer to the individual product labels for appropriate dosing in children.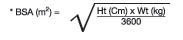
Figure 1 )7.3 Established and Other Potentially Significant Drug InteractionsTable 12 provides a listing of established or potentially clinically significant drug interactions. Alteration in dose or regimen may be recommended based on drug interaction studies or predicted interaction
[see Contraindications (4), Warnings and Precautions (5.1), Clinical Pharmacology (12.3)]for magnitude of interaction.Table 12. Established and Other Potentially Significant Drug Interactions Concomitant Drug Class:Drug NameEffect on Concentration of Lopinavir or Concomitant DrugClinical CommentsHIV-1 Antiviral AgentsHIV-1 Protease Inhibitor: fosamprenavir/ritonavir ↓ amprenavir
↓ lopinavirAn increased rate of adverse reactions has been observed with co-administration of these medications. Appropriate doses of the combinations with respect to safety and efficacy have not been established. HIV-1 Protease Inhibitor: indinavir* ↑ indinavir Decrease indinavir dose to 600 mg twice daily, when co-administered with lopinavir and ritonavir 400/100 mg twice daily. Lopinavir and ritonavir once daily has not been studied in combination with indinavir. HIV-1 Protease Inhibitor: nelfinavir* ↑ nelfinavir
↑ M8 metabolite of nelfinavir
↓ lopinavirLopinavir and ritonavir once daily in combination with nelfinavir is not recommended [see Dosage and Administration (2)].HIV-1 Protease Inhibitor: ritonavir* ↑ lopinavir Appropriate doses of additional ritonavir in combination with lopinavir and ritonavir with respect to safety and efficacy have not been established. HIV-1 Protease Inhibitor: saquinavir ↑ saquinavir The saquinavir dose is 1,000 mg twice daily, when co-administered with lopinavir and ritonavir 400/100 mg twice daily. Lopinavir and ritonavir once daily has not been studied in combination with saquinavir. HIV-1 Protease Inhibitor: tipranavir* ↓ lopinavir Co-administration with tipranavir (500 mg twice daily) and ritonavir (200 mg twice daily) is not recommended. HIV CCR5 – Antagonist: maraviroc* ↑ maraviroc When co-administered, patients should receive 150 mg twice daily of maraviroc. For further details see complete prescribing information for maraviroc. Non-nucleoside Reverse Transcriptase Inhibitors: efavirenz*,
nevirapine*↓ lopinavir Increase the dose of lopinavir and ritonavir tablets to 500/125 mg when lopinavir and ritonavir tablet is co-administered with efavirenz or nevirapine. Lopinavir and ritonavir once daily in combination with efavirenz or nevirapine is not recommended [see Dosage and Administration (2)].Non-nucleoside Reverse Transcriptase Inhibitor: delavirdine ↑ lopinavir Appropriate doses of the combination with respect to safety and efficacy have not been established. Nucleoside Reverse Transcriptase Inhibitor: didanosine Lopinavir and ritonavir tablets can be administered simultaneously with didanosine without food.
For lopinavir and ritonavir oral solution, it is recommended that didanosine be administered on an empty stomach; therefore, didanosine should be given one hour before or two hours after lopinavir and ritonavir oral solution (given with food).Nucleoside Reverse Transcriptase Inhibitor: tenofovir disoproxil fumarate* ↑ tenofovir Patients receiving lopinavir and ritonavir and tenofovir should be monitored for adverse reactions associated with tenofovir. Nucleoside Reverse Transcriptase Inhibitors: abacavir
zidovudine↓ abacavir
↓ zidovudineThe clinical significance of this potential interaction is unknown. Other AgentsAlpha 1- Adrenoreceptor
Antagonist:
alfuzosin↑ alfuzosin Contraindicated due to potential hypotension [see Contraindications (4)].Antianginal:
ranolazine↑ ranolazine Contraindicated due to potential for serious and/or life-threatening reactions [see.Contraindications (4)]Antiarrhythmics:
dronedarone↑ dronedarone Contraindicated due to potential for cardiac arrhythmias [seeContraindications (4)].Antiarrhythmics e.g. amiodarone,
bepridil,
lidocaine (systemic),
quinidine↑ antiarrhythmics Caution is warranted and therapeutic concentration monitoring (if available) is recommended for antiarrhythmics when co-administered with lopinavir and ritonavir. Anticancer Agents:
abemaciclib,
apalutamide,
encorafenib,
ibrutinib,
ivosidenib,
dasatinib,
neratinib,
nilotinib,
venetoclax,
vinblastine,
vincristine↑ anticancer agents
↓lopinavir/ritonavir#Apalutamide is contraindicated due to potential for loss of virologic response and possible resistance to lopinavir and ritonavir or to the class of protease inhibitors [seeContraindications (4)].
Avoid co-administration of encorafenib or ivosidenib with lopinavir and ritonavir due to potential risk of serious adverse events such as QT interval prolongation. If co-administration of encorafenib with lopinavir and ritonavir cannot be avoided, modify dose as recommended in encorafenib USPI. If co-administration of ivosidenib with lopinavir and ritonavir cannot be avoided, reduce ivosidenib dose to 250 mg once daily.Avoid use of neratinib, venetoclax or ibrutinib with lopinavir and ritonavir.
For vincristine and vinblastine, consideration should be given to temporarily withholding the ritonavir-containing antiretroviral regimen in patients who develop significant hematologic or gastrointestinal side effects when lopinavir and ritonavir is administered concurrently with vincristine or vinblastine. If the antiretroviral regimen must be withheld for a prolonged period, consideration should be given to initiating a revised regimen that does not include a CYP3A or P-gp inhibitor.A decrease in the dosage or an adjustment of the dosing interval of nilotinib and dasatinib may be necessary for patients requiring co-administration with strong CYP3A inhibitors such as lopinavir and ritonavir. Please refer to the nilotinib and dasatinib prescribing information for dosing instructions. Anticoagulants:
warfarin,
rivaroxaban↑↓ warfarin
↑ rivaroxabanConcentrations of warfarin may be affected. Initial frequent monitoring of the INR during lopinavir and ritonavir and warfarin co-administration is recommended.
Avoid concomitant use of rivaroxaban and lopinavir and ritonavir. Co-administration of lopinavir and ritonavir and rivaroxaban may lead to increased risk of bleeding.Anticonvulsants:
carbamazepine,
phenobarbital,
phenytoin↓ lopinavir
↓ phenytoinLopinavir and ritonavir may be less effective due to decreased lopinavir plasma concentrations in patients taking these agents concomitantly and should be used with caution.
Lopinavir and ritonavir once daily in combination with carbamazepine, phenobarbital, or phenytoin is not recommended.
In addition, co-administration of phenytoin and lopinavir and ritonavir may cause decreases in steady-state phenytoin concentrations. Phenytoin levels should be monitored when co-administering with lopinavir and ritonavir.Anticonvulsants: lamotrigine,
valproate↓ lamotrigine
↓ or ↔ valproateA dose increase of lamotrigine or valproate may be needed when co-administered with lopinavir and ritonavir and therapeutic concentration monitoring for lamotrigine may be indicated; particularly during dosage adjustments. Antidepressant: bupropion ↓ bupropion
↓ active metabolite,
hydroxybupropionPatients receiving lopinavir and ritonavir and bupropion concurrently should be monitored for an adequate clinical response to bupropion. Antidepressant: trazodone ↑ trazodone Adverse reactions of nausea, dizziness, hypotension and syncope have been observed following co-administration of trazodone and ritonavir. A lower dose of trazodone should be considered. Anti-infective: clarithromycin ↑ clarithromycin For patients with renal impairment, adjust clarithromycin dose as follows: - For patients on lopinavir and ritonavir with CLCR30 to 60 mL/min the dose of clarithromycin should be reduced by 50%.
- For patients on lopinavir and ritonavir with CLCR< 30 mL/min the dose of clarithromycin should be decreased by 75%.
No dose adjustment for patients with normal renal function is necessary.Antifungals: ketoconazole*, itraconazole, voriconazole isavuconazonium sulfate* ↑ ketoconazole
↑ itraconazole
↓ voriconazole
↑ isavuconazoniumHigh doses of ketoconazole (>200 mg/day) or itraconazole (> 200 mg/day) are not recommended.
The coadministration of voriconazole and lopinavir and ritonavir should be avoided unless an assessment of the benefit/risk to the patient justifies the use of voriconazole.
Isavuconazonium and lopinavir and ritonavir should be coadministered with caution. Alternative antifungal therapies should be considered in these patients.Anti-gout: colchicine ↑ colchicine Contraindicated due to potential for serious and/or life-threatening reactions in patients with renal and/or hepatic impairment [see.Contraindications (4)]For patients with normal renal or hepatic function:Treatment of gout flares-co-administration of colchicine in patients onlopinavir and ritonavir:
0.6 mg (1 tablet) x 1 dose, followed by 0.3 mg (half tablet) 1 hour later. Dose to be repeated no earlier than 3 days.Prophylaxis of gout flares-co-administration of colchicine in patients onlopinavir and ritonavir:
If the original colchicine regimen was 0.6 mg twice a day, the regimen should be adjusted to 0.3 mg once a day.If the original colchicine regimen was 0.6 mg once a day, the regimen should be adjusted to 0.3 mg once every other day. Treatment of familial Mediterranean fever (FMF)-co-administration of colchicine in patients onlopinavir and ritonavir:
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day).Antimycobacterial:
rifampin↓ lopinavir Contraindicated due to potential loss of virologic response and possible resistance to lopinavir and ritonavir or to the class of protease inhibitors or other co-administered antiretroviral agents [seeContraindications (4)].Antimycobacterial: bedaquiline ↑ bedaquiline Bedaquiline should only be used with lopinavir and ritonavirif the benefit of co-administration outweighs the risk. Antimycobacterial: rifabutin* ↑ rifabutin and rifabutin metabolite Dosage reduction of rifabutin by at least 75% of the usual dose of 300 mg/day is recommended (i.e., a maximum dose of 150 mg every other day or three times per week). Increased monitoring for adverse reactions is warranted in patients receiving the combination. Further dosage reduction of rifabutin may be necessary. Antiparasitic: atovaquone ↓ atovaquone Clinical significance is unknown; however, increase in atovaquone doses may be needed. Antipsychotics:
lurasidone
pimozide↑ lurasidone
↑ pimozideContraindicated due to potential for serious and/or life-threatening reactions [seeContraindications (4)].
Contraindicated due to potential for serious and/or life-threatening reactions such as cardiac
arrhythmias[seeContraindications (4)].Antipsychotics: quetiapine ↑ quetiapine Initiation of lopinavir and ritonavir in patients taking quetiapine:
Consider alternative antiretroviral therapy to avoid increases in quetiapine exposures. If coadministration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring.Initiation of quetiapine in patients taking lopinavir and ritonavir:
Refer to the quetiapine prescribing information for initial dosing and titration of quetiapine.Contraceptive:
ethinyl estradiol*↓ ethinyl estradiol Because contraceptive steroid concentrations may be altered when lopinavir and ritonavir is co- administered with oral contraceptives or with the contraceptive patch, alternative methods of nonhormonal contraception are recommended. Dihydropyridine Calcium
Channel Blockers: e.g.
felodipine,
nifedipine,
nicardipine↑ dihydropyridine
calcium channel
blockersClinical monitoring of patients is recommended and a dose reduction of the dihydropyridine calcium channel blocker may be considered. Disulfiram/metronidazole Lopinavir and ritonavir oral solution contains ethanol, which can produce disulfiram-like reactions when co-administered with disulfiram or other drugs that produce this reaction (e.g., metronidazole). Endothelin Receptor Antagonists: bosentan ↑ bosentan Co-administration of bosentan in patients on lopinavir and ritonavir:
In patients who have been receiving lopinavir and ritonavir for at least 10 days, start bosentan at 62.5 mg once daily or every other day based upon individual tolerability.Co-administration of lopinavir and ritonavir in patients on bosentan:
Discontinue use of bosentan at least 36 hours prior to initiation of lopinavir and ritonavir.
After at least 10 days following the initiation of lopinavir and ritonavir, resume bosentan at 62.5 mg once daily or every other day based upon individual tolerability.Ergot Derivatives:
dihydroergotamine,
ergotamine,
methylergonovine↑ ergot derivatives Contraindicated due to potential for acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues [seeContraindications (4)].GI Motility Agent:
cisapride↑ cisapride Contraindicated due to potential for cardiac arrhythmias [seeContraindications (4)].GnRH Receptor Antagonists: elagolix ↑ elagolix
↓ lopinavir/ritonavirConcomitant use of elagolix 200 mg twice daily and lopinavir and ritonavir for more than 1 month is not recommended due to potential risk of adverse events such as bone loss and hepatic transaminase elevations. Limit concomitant use of elagolix 150 mg once daily and lopinavir and ritonavir to 6 months. Hepatitis C direct acting antiviral: elbasvir/grazoprevir ↑ elbasvir/grazoprevir Contraindicated due to increased risk of alanine transaminase (ALT) elevations [seeContraindications (4)].Hepatitis C direct acting antivirals:
boceprevir*
glecaprevir/Pibrentasvir
simeprevir
sofosbuvir/velpatasvir/voxilaprevi
r
ombitasvir/paritaprevir/ ritonavir and dasabuvir*↓ lopinavir
↓ boceprevir
↓ ritonavir
↑ glecaprevir
↑ pibrentasvir
↑ simeprevir
↑ sofosbuvir
↑ velpatasvir
↑ voxilaprevir
↑ ombitasvir
↑ paritaprevir
↑ ritonavir
↔ dasabuvirIt is not recommended to co-administer lopinavir and ritonavir and boceprevir, glecaprevir/pibrentasvir, simeprevir, sofosbuvir/velpatasvir/voxilaprevir,or ombitasvir/paritaprevir/ritonavir and dasabuvir. Herbal Products:
St. John's Wort (hypericum
perforatum)↓ lopinavir Contraindicated due to potential for loss of virologic response and possible resistance to lopinavir and ritonavir or to the class of protease inhibitors [seeContraindications (4)].Lipid-modifying agents
HMG-CoA Reductase Inhibitors:
lovastatin
simvastatin
atorvastatin
rosuvastatin
Microsomal triglyceride transfer
protein (MTTP) Inhibitor:
lomitapide
↑ lovastatin
↑ simvastatin
↑ atorvastatin
↑ rosuvastatin
↑ lomitapide
Contraindicated due to potential for myopathy including rhabdomyolysis[seeContraindications (4)].
Use atorvastatin with caution and at the lowest necessary dose. Titrate rosuvastatin dose carefully and use the lowest necessary dose; do not exceed rosuvastatin 10 mg/day.
Lomitapide is a sensitive substrate for CYP3A4 metabolism. CYP3A4 inhibitors increase the
exposure of lomitapide, with strong inhibitors increasing exposure approximately 27-fold.
Concomitant use of moderate or strong CYP3A4 inhibitors with lomitapide is contraindicated due to potential for hepatotoxicity[see.Contraindications (4)]Immunosuppressants: e.g.
cyclosporine,
tacrolimus,
sirolimus↑ immunosuppressants Therapeutic concentration monitoring is recommended for immunosuppressant agents when co-administered with lopinavir and ritonavir. Kinase Inhibitors:
fostamatinib(also see anticancer agents above)↑ fostamatinib
metabolite R406Monitor for toxicities of R406 such as hepatotoxicity and neutropenia. Fostamatinib dose reduction may be required. Long-acting beta-adrenoceptor Agonist:
salmeterol↑ salmeterol Concurrent administration of salmeterol and lopinavir and ritonavir is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations and sinus tachycardia. Narcotic Analgesics: methadone,*
fentanyl↓ methadone
↑ fentanylDosage of methadone may need to be increased when co-administered with lopinavir and ritonavir.
Careful monitoring of therapeutic and adverse effects (including potentially fatal respiratory depression) is recommended when fentanyl is concomitantly administered with lopinavir and ritonavir.PDE5 inhibitors: avanafil,
sildenafil,
tadalafil,
vardenafil↑ avanafil
↑ sildenafil
↑ tadalafil
↑ vardenafilSildenafil when used for the treatment of pulmonary arterial hypertension (Revatio®) is contraindicated due to the potential for sildenafil-associated adverse events, including visual abnormalities, hypotension, prolonged erection, and syncope [see.Contraindications(4)]
Do not use lopinavir and ritonavir with avanafil because a safe and effective avanafil dosage regimen has not been established.
Particular caution should be used when prescribing sildenafil, tadalafil, or vardenafil in patients receiving lopinavir and ritonavir. Co-administration of lopinavir and ritonavir with these drugs may result in an increase in PDE5 inhibitor associated adverse reactions including hypotension, syncope, visual changes and prolonged erection.
Use of PDE5 inhibitors for pulmonary arterial hypertension (PAH):
Sildenafil (Revatio®) is contraindicated[see.Contraindications (4)]
The following dose adjustments are recommended for use of tadalafil (Adcirca®) with lopinavir and ritonavir:Co-administration of ADCIRCA in patients on lopinavir and ritonavir:
In patients receiving lopinavir and ritonavir for at least one week, start ADCIRCA at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability.Co-administration of lopinavir and ritonavir in patients on ADCIRCA:
Avoid use of ADCIRCA during the initiation of lopinavir and ritonavir. Stop ADCIRCA at least 24 hours prior to starting lopinavir and ritonavir. After at least one week following the initiation of lopinavir and ritonavir, resume ADCIRCA at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability.
Use of PDE5 inhibitors for erectile dysfunction:
It is recommended not to exceed the following doses:- Sildenafil: 25 mg every 48 hours
- Tadalafil: 10 mg every 72 hours
- Vardenafil: 2.5 mg every 72 hours
Sedative/Hypnotics:
triazolam,
orally administered midazolam↑ triazolam
↑ midazolamContraindicated due to potential for prolonged or increased sedation or respiratory depression [seeContraindications (4)].Sedative/Hypnotics:
parenterally administered midazolam↑ midazolam If lopinavir and ritonavir is co-administered with parenteral midazolam, close clinical monitoring for respiratory depression and/or prolonged sedation should be exercised and dosage adjustment should be considered. Systemic/Inhaled/
Nasal/Ophthalmic
Corticosteroids: e.g.,
betamethasone
budesonide
ciclesonide
dexamethasone
fluticasone
methylprednisolone
mometasone
prednisone
triamcinolone↓ lopinavir
↑ glucocorticoidsCoadministration with oral dexamethasone or other systemic corticosteroids that induce CYP3A may result in loss of therapeutic effect and development of resistance to lopinavir. Consider alternative corticosteroids.
Coadministration with corticosteroids whose exposures are significantly increased by strong CYP3A inhibitors can increase the risk for Cushing’s syndrome and adrenal suppression.
Alternative corticosteroids including beclomethasone and prednisolone (whose PK and/or PD are less affected by strong CYP3A inhibitors relative to other studied steroids) should be considered, particularly for long-term use.* seefor magnitude of interaction.Clinical Pharmacology (12.3)
#refers to interaction with apalutamide. - Adult patients with three or more of the following lopinavir resistance-associated substitutions: L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V, I54L/T/V, V82A/C/F/S/T, and I84V
- Lopinavir and ritonavir oral solution should not be administered to neonates before a postmenstrual age (first day of the mother’s last menstrual period to birth plus the time elapsed after birth) of 42 weeks and a postnatal age of at least 14 days has been attained (,
2.4 Dosage Recommendations in Pediatric PatientsLopinavir and ritonavir tablets and oral solution are not recommended for once daily dosing in pediatric patients younger than 18 years of age. The dose of the oral solution should be administered using the calibrated cup (supplied) or oral dosing syringe. Lopinavir and ritonavir 100/25 mg tablets should be considered only in children who have reliably demonstrated the ability to swallow the intact tablet.
Lopinavir and ritonavir oral solution is not recommended in neonates before a postmenstrual age (first day of the mother’s last menstrual period to birth plus the time elapsed after birth) of 42 weeks and a postnatal age of at least 14 days has been attained
[seeWarnings and Precautions (5.2)].Lopinavir and ritonavir oral solution contains approximately 42% (v/v) ethanol and approximately 15% (w/v) propylene glycol. Total amounts of ethanol and propylene glycol from all medicines that are to be given to pediatric patients 14 days to 6 months of age should be taken into account in order to avoid toxicity from these excipients
[see Warnings and Precautions (5.2)andOverdosage (10)].Pediatric Dosage CalculationsCalculate the appropriate dose of lopinavir and ritonavir for each individual pediatric patient based on body weight (kg) or body surface area (BSA) to avoid underdosing or exceeding the recommended adult dose.
Body surface area (BSA) can be calculated as follows:
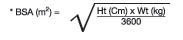
The lopinavir and ritonavir dose can be calculated based on weight or BSA:
Based on Weight:Patient Weight (kg) × Prescribed lopinavir dose (mg/kg) = Administered lopinavir dose (mg)
Based on BSA:Patient BSA (m2) × Prescribed lopinavir dose (mg/m2) = Administered lopinavir dose (mg)
If lopinavir and ritonavir oral solution is used, the volume (mL) of lopinavir and ritonavir solution can be determined as follows:
Volume of lopinavir and ritonavir solution (mL) = Administered lopinavir dose (mg) ÷ 80 (mg/mL)
Oral Solution Dosage Recommendation in Pediatric Patients 14 Days to Less Than 18 Years:Table 4 summarizes the recommended daily dosing regimen for pediatric patients 14 days to less than 18 years of age using the oral solution.
Lopinavir and ritonavir administered in combination with efavirenz, nevirapine, or nelfinavir in patients younger than 6 months of age is not recommended. Total dose of lopinavir and ritonavir oral solution in pediatric patients should not exceed the recommended adult daily dose of 400/100 mg (5mL) twice daily.
Table 4. Lopinavir and Ritonavir Oral Solution Daily Dosage Recommendations in Pediatric Patients 14 days to Less Than 18 Years Without Concomitant Efavirenz, Nevirapine, or Nelfinavir Patient AgeBased on Weight(mg/kg)Based on BSA(mg/m2)Frequency14 days to 6 months 16/4 300/75 Given twice
dailyOlder than 6 months to less than 18 years Less than 15 kg 12/3 230/57.5 Given twice
daily15 kg to 40 kg 10/2.5 Tablet Dosage Recommendation in Pediatric Patients Older than 6 Months to Less than 18 Years:Table 5 provides the dosing recommendations for pediatric patients older than 6 months to less than 18 years of age based on body weight or body surface area for lopinavir and ritonavir tablets.
Table 5. Lopinavir and Ritonavir Tablet Daily Dosage Recommendations in Pediatric Patients > 6 Months to < 18 Years of Age Without Concomitant Efavirenz, Nevirapine, or Nelfinavir Body Weight (kg)Body Surface Area (m2)*Recommended numberof 100/25 mg TabletsTwice Daily≥15 to 25 ≥0.6 to < 0.9 2 >25 to 35 ≥0.9 to < 1.4 3 >35 ≥1.4 4 * Lopinavir and ritonavir oral solution is available for children with a BSA less than 0.6 m2or those who are unable to reliably swallow a tablet. Concomitant Therapy: Efavirenz, Nevirapine, or NelfinavirDosing recommendations using oral solutionTable 6 provides the dosing recommendations for pediatric patients older than 6 months to less than 18 years of age based on body weight or body surface area for Lopinavir and Ritonavir Oral Solution when given in combination with efavirenz, nevirapine, or nelfinavir:
Table 6. Lopinavir and Ritonavir Oral Solution Daily Dosage Recommendations for Pediatric Patients >6 Months to < 18 Years of Age With Concomitant Efavirenz, Nevirapine, or Nelfinavir Patient AgeBased on Weight(mg/kg)Based on BSA(mg/m2)Frequency> 6 months to
< 18 years<15 kg 13/3.25 300/75 Given twice daily ≥15 kg to 45 kg 11/2.75 Dosing recommendations using tabletsTable 7 provides the dosing recommendations for pediatric patients older than 6 months to less than 18 years of age based on body weight or body surface area for lopinavir and ritonavir tablets when given in combination with efavirenz, nevirapine, or nelfinavir.
Table 7. Lopinavir and Ritonavir Tablet Daily Dosage Recommendations for Pediatric Patients > 6 Months to < 18 Years of Age With Concomitant Efavirenz†, Nevirapine, or Nelfinavir† Body Weight (kg)Body Surface Area (m2)*Recommended number of 100/25 mg Tablets Twice Daily≥15 to 20 ≥0.6 to < 0.8 2 >20 to 30 ≥0.8 to < 1.2 3 >30 to 45 ≥1.2 to <1.7 4 >45 ≥1.7 5 [see Dosage and Administration (2.4)]* Lopinavir and ritonavir oral solution is available for children with a BSA less than 0.6 m2or those who are unable to reliably swallow a tablet. †Please refer to the individual product labels for appropriate dosing in children.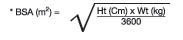
Figure 1 )5.1 Risk of Serious Adverse Reactions Due to Drug InteractionsInitiation of lopinavir and ritonavir, a CYP3A inhibitor, in patients receiving medications metabolized by CYP3A or initiation of medications metabolized by CYP3A in patients already receiving lopinavir and ritonavir, may increase plasma concentrations of medications metabolized by CYP3A. Initiation of medications that inhibit or induce CYP3A may increase or decrease concentrations of lopinavir and ritonavir, respectively. These interactions may lead to:
- Clinically significant adverse reactions, potentially leading to severe, life-threatening, or fatal events from greater exposures of concomitant medications.
- Clinically significant adverse reactions from greater exposures of lopinavir and ritonavir.
- Loss of therapeutic effect of lopinavir and ritonavir and possible development of resistance.
See Table 12 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations
[ see Drug Interactions (7)]. Consider the potential for drug interactions prior to and during lopinavir and ritonavir therapy; review concomitant medications during lopinavir and ritonavir therapy, and monitor for the adverse reactions associated with the concomitant medications[ see Contraindications (4)andDrug Interactions (7)].
Administer 400/100 mg of lopinavir and ritonavir twice daily in pregnant patients with no documented lopinavir-associated resistance substitutions.
- Once daily lopinavir and ritonavir dosing is not recommended in pregnancy[ see Use in Specific Populations (8.1)and Clinical Pharmacology (12.3)].
- There are insufficient data to recommend dosing in pregnant women with any documented lopinavir-associated resistance substitutions.
- No dosage adjustment of lopinavir and ritonavir is required for patients during the postpartum period.
- Avoid use of lopinavir and ritonavir oral solution in pregnant women[see Use in Specific Populations (8.1)].
- 400/100 mg twice daily in pregnant patients with no documented lopinavir-associated resistance substitutions.
- There are insufficient data to recommend a lopinavir and ritonavir dose for pregnant patients with any documented lopinavir and ritonavir-associated resistance substitutions.
- No dose adjustment of lopinavir and ritonavir is required for patients during the postpartum period.
Lactation: Breastfeeding not recommended. (
The Centers for Disease Control and Prevention recommend that HIV-1 infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV-1. Because of the potential for: 1) HIV transmission (in HIV-negative infants), 2) developing viral resistance (in HIV-positive infants), and 3) adverse reactions in the breastfed infant, instruct mothers not to breastfeed if they are receiving lopinavir and ritonavir.
- Lopinavir and ritonavir is contraindicated in patients with previously demonstrated clinically significant hypersensitivity (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome, erythema multiforme, urticaria, angioedema) to any of its ingredients, including ritonavir.
- Lopinavir and ritonavir is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening reactions [see.and
7.1 Potential for Lopinavir and Ritonavir to Affect Other DrugsLopinavir/ritonavir is an inhibitor of CYP3A and may increase plasma concentrations of agents that are primarily metabolized by CYP3A. Agents that are extensively metabolized by CYP3A and have high first pass metabolism appear to be the most susceptible to large increases in AUC (>3-fold) when co-administered with lopinavir and ritonavir. Thus, co-administration of lopinavir and ritonavir with drugs highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated. Co-administration with other CYP3A substrates may require a dose adjustment or additional monitoring as shown in Table 12.
Additionally, lopinavir and ritonavir induces glucuronidation.
Published data suggest that lopinavir is an inhibitor of OATP1B1.
These examples are a guide and not considered a comprehensive list of all possible drugs that may interact with lopinavir/ritonavir. The healthcare provider should consult appropriate references for comprehensive information.
]12.3 PharmacokineticsThe pharmacokinetic properties of lopinavir are summarized in Table 13. The steady-state pharmacokinetic parameters of lopinavir are summarized in Table 14. Under fed conditions, lopinavir concentrations were similar following administration of lopinavir and ritonavir tablets to capsules with less pharmacokinetic variability. Under fed conditions (500 kcal, 25% from fat), lopinavir concentrations were similar following administration of lopinavir and ritonavir capsules and oral solution.
Table 13. Pharmacokinetic Properties of Lopinavir AbsorptionTmax(hr)a 4.4 ± 0.8 Effect of meal
(relative to fasting)
Tablet
Oral solution
↑ 19%b
↑ 130%bDistribution% Bound to human plasma proteins > 98 Vd/Fa(L) 16.9 MetabolismMetabolism CYP3A EliminationMajor route of elimination hepatic t1/2(h)a 6.9 ± 2.2 % of dose excreted in urine 10.4 ± 2.3 % of dose excreted in feces 82.6 ± 2.5 a.Lopinavir and ritonavir tablet
b.Changes in AUC valuesTable 14. Steady-State Pharmacokinetic Parameters of Lopinavir, Mean ± SD Pharmacokinetic ParameterTwice DailyaOnce DailybCmax(mcg/mL) 9.8 ± 3.7 11.8 ± 3.7 Cmin(mcg/mL) 5.5 ± 2.7 1.7 ± 1.6 AUCtau(mcg•h/mL) 92.6 ± 36.7 154.1 ± 61.4 a. 19 HIV-1 subjects, lopinavir and ritonavir 400/100 mg twice daily
b. 24 HIV-1 subjects, lopinavir and ritonavir 800/200 mg + emtricitabine 200 mg + tenofovir DF 300 mgSpecific PopulationsGender, Race and AgeNo gender or race related pharmacokinetic differences have been observed in adult patients. Lopinavir pharmacokinetics have not been studied in elderly patients.
Pediatric PatientsThe 230/57.5 mg/m2twice daily regimen without nevirapine and the 300/75 mg/m2twice daily regimen with nevirapine provided lopinavir plasma concentrations similar to those obtained in adult patients receiving the 400/100 mg twice daily regimen without nevirapine.
Table 15. Lopinavir Pharmacokinetic Data from Pediatric Clinical Trials, Mean ± SD Cmax(mcg/mL)Cmin(mcg/mL)AUC12(mcg•hr/m)Age ≥ 14 Days to < 6 Weeks Cohort (N = 9):5.17 ± 1.84a 1.40 ± 0.48a 43.39 ± 14.80a Age ≥ 6 Weeks to < 6 Months Cohort (N = 18):9.39 ± 4.91a 1.95 ± 1.80a 74.50 ± 37.87a Age ≥ 6 Months to ≤ 12 years Cohort (N = 24):8.2 ± 2.9b 3.4 ± 2.1b 72.6 ± 31.1b 10.0 ± 3.3c 3.6 ± 3.5c 85.8 ± 36.9c a. Lopinavir and ritonavir oral solution 300/75 mg/m2twice daily without concomitant NNRTI therapy
b. Lopinavir and ritonavir oral solution 230/57.5 mg/m2twice daily without nevirapine (n=12)
c. Lopinavir and ritonavir oral solution 300/75 mg/m2twice daily with nevirapine (n=12)PregnancyThe C12hvalues of lopinavir were lower during the second and third trimester by approximately 40% as compared to post-partum in 12 HIV-infected pregnant women received lopinavir and ritonavir 400 mg/100 mg twice daily. Yet this decrease is not considered clinically relevant in patients with no documented lopinavir and ritonavir-associated resistance substitutions receiving 400 mg/100 mg twice daily
[see Use in Specific Populations (8.1)].Renal ImpairmentLopinavir pharmacokinetics have not been studied in patients with renal impairment; however, since the renal clearance of lopinavir is negligible, a decrease in total body clearance is not expected in patients with renal impairment.
Hepatic ImpairmentMultiple dosing of lopinavir and ritonavir 400/100 mg twice daily to HIV-1 and HCV co-infected patients with mild to moderate hepatic impairment (n = 12) resulted in a 30% increase in lopinavir AUC and 20% increase in Cmaxcompared to HIV-1 infected subjects with normal hepatic function (n = 12). Additionally, the plasma protein binding of lopinavir was statistically significantly lower in both mild and moderate hepatic impairment compared to controls (99.09 vs. 99.31%, respectively). Lopinavir and ritonavir has not been studied in patients with severe hepatic impairment
[see Warnings and Precautions (5.4)andUse in Specific Populations (8.6)].Drug InteractionsLopinavir and ritonavir is an inhibitor of the P450 isoform CYP3A
in vitro. Lopinavir and ritonavir does not inhibit CYP2D6, CYP2C9, CYP2C19, CYP2E1, CYP2B6 or CYP1A2 at clinically relevant concentrations.Lopinavir and ritonavir has been shown
in vivoto induce its own metabolism and to increase the biotransformation of some drugs metabolized by cytochrome P450 enzymes and by glucuronidation.The effects of co-administration of lopinavir and ritonavir on the AUC, Cmaxand Cminare summarized in Table 16 (effect of other drugs on lopinavir) and Table 17 (effect of lopinavir and ritonavir on other drugs). For information regarding clinical recommendations, see Table 12 in Drug Interactions (7).
Table 16. Drug Interactions: Pharmacokinetic Parameters for Lopinavir in the Presence of the Co-administered Drug for Recommended Alterations in Dose or Regimen Co- administered DrugDose of Co- administered Drug(mg)Dose ofLopinavir and Ritonavir(mg)nRatio (in combination with Co-administered drug/alone) of Lopinavir Pharmacokinetic Parameters (90% CI);No Effect = 1.00CmaxAUCCminEfavirenz1 600 at bedtime 400/100 capsule twice daily 11, 73 0.97
(0.78, 1.22)0.81
(0.64, 1.03)0.61
(0.38, 0.97)600 at bedtime 500/125 tablet twice daily 19 1.12
(1.02, 1.23)1.06
(0.96, 1.17)0.90
(0.78, 1.04)600 at bedtime 600/150 tablet twice daily 23 1.36
(1.28, 1.44)1.36
(1.28, 1.44)1.32
(1.21, 1.44)Etravirine 200 twice daily 400/100 mg twice daily (tablets) 16 0.89
(0.82 to 0.96)0.87
(0.83 to 0.92)0.80
(0.73 to 0.88)Fosamprenavir2 700 twice daily plus ritonavir 100 twice daily 400/100 capsule twice daily 18 1.30
(0.85, 1.47)1.37
(0.80, 1.55)1.52
(0.72, 1.82)Ketoconazole 200 single dose 400/100 capsule twice daily 12 0.89
(0.80, 0.99)0.87
(0.75, 1.00)0.75
(0.55, 1.00)Nelfinavir 1,000 twice daily 400/100 capsule twice daily 13 0.79
(0.70, 0.89)0.73
(0.63, 0.85)0.62
(0.49, 0.78)Nevirapine 200 twice daily steady-state 400/100 capsule twice daily 22, 193 0.81
(0.62, 1.05)0.73
(0.53, 0.98)0.49
(0.28, 0.74)7 mg/kg or 4 mg/kg once daily; twice daily 1 wk5 (> 1 yr) 300/ 75 mg/m2oral solution twice daily 12, 153 0.86
(0.64, 1.16)0.78
(0.56, 1.09)0.45
(0.25, 0.81)Ombitasvir/ paritaprevir/ ritonavir+ dasabuvir2 25/150/100 +
dasabuvir 400400/100 tablet twice daily 6 0.87
(0.76, 0.99)0.94
(0.81, 1.10)1.15
(0.93, 1.42)Omeprazole 40 once daily, 5 d 400/100 tablet twice daily, 10 d 12 1.08
(0.99, 1.17)1.07
(0.99, 1.15)1.03
(0.90, 1.18)40 once daily, 5 d 800/200 tablet once daily, 10 d 12 0.94
(0.88, 1.00)0.92
(0.86, 0.99)0.71
(0.57, 0.89)Pravastatin 20 once daily, 4 d 400/100 capsule twice daily, 14 d 12 0.98
(0.89, 1.08)0.95
(0.85, 1.05)0.88
(0.77, 1.02)Ranitidine 150 single dose 400/100 tablet twice daily, 10 d 12 0.99
(0.95, 1.03)0.97
(0.93, 1.01)0.90
(0.85, 0.95)150 single dose 800/200 tablet once daily, 10 d 10 0.97
(0.95, 1.00)0.95
(0.91, 0.99)0.82
(0.74, 0.91)Rifabutin 150 once daily 400/100 capsule twice daily 14 1.08
(0.97, 1.19)1.17
(1.04, 1.31)1.20
(0.96, 1.65)Rifampin 600 once daily 400/100 capsule twice daily 22 0.45
(0.40, 0.51)0.25
(0.21, 0.29)0.01
(0.01, 0.02)600 once daily 800/200 capsule twice daily 10 1.02
(0.85, 1.23)0.84
(0.64, 1.10)0.43
(0.19, 0.96)600 once daily 400/400 capsule twice daily 9 0.93
(0.81, 1.07)0.98
(0.81, 1.17)1.03
(0.68, 1.56)Rilpivirine 150 once daily 400/100 twice daily (capsules) 15 0.96
(0.88 to 1.05)0.99
(0.89 to 1.10)0.89
(0.73 to 1.08)Ritonavir 100 twice daily 400/100 capsule twice daily 8, 213 1.28
(0.94, 1.76)1.46
(1.04, 2.06)2.16
(1.29, 3.62)Tipranavir/ ritonavir 500/200 twice daily 400/100 capsule twice daily 21, 693 0.53
(0.40, 0.69)0.45
(0.32, 0.63)0.30
(0.17, 0.51)
0.484
(0.40, 0.58)1.000000000000000e+00Reference for comparison is lopinavir/ritonavir 400/100 mg twice daily without efavirenz.
2.000000000000000e+00Data extracted from the U.S. prescribing information of co-administered drugs.
3.000000000000000e+00Parallel group design
4.000000000000000e+00Drug levels obtained at 8 to 16 hours post dose
N/A = Not available.Table 17. Drug Interactions: Pharmacokinetic Parameters for Co-administered Drug in the Presence of Lopinavir and Ritonavir for Recommended Alterations in Dose or Regimen Co- administered DrugDose of Co- administered Drug (mg)Dose ofLopinavir and Ritonavir(mg)nRatio (in combination withLopinavir and Ritonavir/alone) of Co-administered Drug Pharmacokinetic Parameters (90% CI); No Effect = 1.00CmaxAUCCminBedaquiline1 400 single dose 400/100 twice daily N/A N/A 1.22
(1.11, 1.34)N/A Efavirenz 600 at bedtime 400/100
capsule twice daily11, 123 0.91
(0.72, 1.15)0.84
(0.62, 1.15)0.84
(0.58, 1.20)Elbasvir/ grazoprevir1 50 once daily 400/100 twice daily 10 2.87
(2.29, 3.58)3.71
(3.05, 4.53)4.58
(3.72, 5.64)200 once daily 13 7.31
(5.65, 9.45)12.86
(10.25, 16.13)21.70
(12.99, 36.25)Ethinyl Estradiol 35 mcg once daily
(Ortho Novum®)400/100
capsule twice daily12 0.59
(0.52, 0.66)0.58
(0.54, 0.62)0.42
(0.36, 0.49)Etravirine 200 twice daily 400/100 tablet twice day 16 0.70
(0.64 to 0.78)0.65
(0.59 to 0.71)0.55
(0.49 to 0.62)Fosamprenavir1 700 twice daily plus ritonavir 100 twice daily 400/100
capsule twice daily18 0.42
(0.30, 0.58)0.37
(0.28, 0.49)0.35
(0.27, 0.46)Indinavir 600 twice daily combo nonfasting vs. 800 three times daily alone fasting 400/100
capsule twice daily13 0.71
(0.63, 0.81)0.91
(0.75, 1.10)3.47
(2.60, 4.64)Ketoconazole 200 single dose 400/100
capsule twice daily12 1.13
(0.91, 1.40)3.04
(2.44, 3.79)N/A Maraviroc1 300 twice daily 400/100
twice daily11 1.97
(1.66, 2.34)3.95
(3.43, 4.56)9.24
(7.98, 10.7)Methadone 5 single dose 400/100
capsule twice daily11 0.55
(0.48, 0.64)0.47
(0.42, 0.53)N/A Nelfinavir 1,000 twice daily combo vs.
1,250 twice daily alone400/100
capsule twice daily13 0.93
(0.82, 1.05)1.07
(0.95, 1.19)1.86
(1.57, 2.22)M8 metabolite 2.36
(1.91, 2.91)3.46
(2.78, 4.31)7.49
(5.85, 9.58)Nevirapine 200 once daily twice daily 400/100
capsule twice daily5, 63 1.05
(0.72, 1.52)1.08
(0.72, 1.64)1.15
(0.71, 1.86)Norethindrone 1 once daily
(Ortho Novum®)400/100
capsule twice daily12 0.84
(0.75, 0.94)0.83
(0.73, 0.94)0.68
(0.54, 0.85)Ombitasvir/ paritaprevir/ ritonavir+ dasabuvir1 25/150/100 +
dasabuvir 400400/100
tablet twice
daily6 1.14
(1.01, 1.28)1.17
(1.07, 1.28)1.24
(1.14, 1.34)2.04
(1.30, 3.20)2.17
(1.63, 2.89)2.36
(1.00, 5.55)1.55
(1.16, 2.09)2.05
(1.49, 2.81)5.25
(3.33, 8.28)0.99
(0.75, 1.31)0.93
(0.75, 1.15)0.68
(0.57, 0.80)Pitavastatin1 4 once daily 400/100 tablet twice daily 23 0.96
(0.84 to 1.10)0.80
(0.73 to 0.87)N/A Pravastatin 20 once daily 400/100 capsule twice daily 12 1.26
(0.87, 1.83)1.33
(0.91, 1.94)N/A Rifabutin 150 once daily
combo vs. 300
once daily
alone400/100 capsule twice daily 12 2.12
(1.89, 2.38)3.03
(2.79, 3.30)4.90
(3.18, 5.76)25- O-desacetyl rifabutin23.6
(13.7, 25.3)47.5
(29.3, 51.8)94.9
(74.0, 122)Rifabutin + 25- O-desacetyl rifabutin3.46
(3.07, 3.91)5.73
(5.08, 6.46)9.53
(7.56, 12.01)Rilpivirine 150 once daily 400/100 capsules twice daily 15 1.29
(1.18 to 1.40)1.52
(1.36 to 1.70)1.74
(1.46 to 2.08)Rosuvastatin2 20 once daily 400/100 tablet twice daily 15 4.66
(3.4, 6.4)2.08
(1.66, 2.6)1.04
(0.9, 1.2)Tenofovir alafenamide1 10 once daily 800/200
tablet once daily10 2.19
(1.72, 2.79)1.47
(1.17, 1.85)N/A Tenofovir disoproxil fumarate1 300 once daily 400/100 capsule twice daily 24 No Change 1.32
(1.26, 1.38)1.51
(1.32, 1.66)1.000000000000000e+00Data extracted from the U.S. prescribing information of co-administered drugs.
2.000000000000000e+00Kiser, et al. J Acquir Immune Defic Syndr. 2008 Apr 15; 47(5):570-8.
3Parallel group design
N/A = Not available.
○ Alpha 1- Adrenoreceptor Antagonist : alfuzosin
○ Antianginal: ranolazine
○ Antiarrhythmic: dronedarone
○ Anti-gout: colchicine
○ Antipsychotics: lurasidone, pimozide
○ Ergot Derivatives: dihydroergotamine, ergotamine, methylergonovine
○ GI Motility Agent: cisapride
○ Hepatitis C direct acting antiviral: elbasvir/grazoprevir
○ HMG-CoA Reductase Inhibitors: lovastatin, simvastatin
○ Microsomal triglyceride
○ PDE5 Inhibitor: sildenafil (Revatio®) when used for the treatment of pulmonary arterial hypertension
○ Sedative/Hypnotics: triazolam, orally administered midazolam
- Lopinavir and ritonavir is contraindicated with drugs that are potent CYP3A inducers where significantly reduced lopinavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance and cross-resistance [seeand
7.2 Potential for Other Drugs to Affect LopinavirLopinavir/ritonavir is a CYP3A substrate; therefore, drugs that induce CYP3A may decrease lopinavir plasma concentrations and reduce lopinavir and ritonavir’s therapeutic effect. Although not observed in the lopinavir and ritonavir/ketoconazole drug interaction study, co-administration of lopinavir and ritonavir and other drugs that inhibit CYP3A may increase lopinavir plasma concentrations.
].12.3 PharmacokineticsThe pharmacokinetic properties of lopinavir are summarized in Table 13. The steady-state pharmacokinetic parameters of lopinavir are summarized in Table 14. Under fed conditions, lopinavir concentrations were similar following administration of lopinavir and ritonavir tablets to capsules with less pharmacokinetic variability. Under fed conditions (500 kcal, 25% from fat), lopinavir concentrations were similar following administration of lopinavir and ritonavir capsules and oral solution.
Table 13. Pharmacokinetic Properties of Lopinavir AbsorptionTmax(hr)a 4.4 ± 0.8 Effect of meal
(relative to fasting)
Tablet
Oral solution
↑ 19%b
↑ 130%bDistribution% Bound to human plasma proteins > 98 Vd/Fa(L) 16.9 MetabolismMetabolism CYP3A EliminationMajor route of elimination hepatic t1/2(h)a 6.9 ± 2.2 % of dose excreted in urine 10.4 ± 2.3 % of dose excreted in feces 82.6 ± 2.5 a.Lopinavir and ritonavir tablet
b.Changes in AUC valuesTable 14. Steady-State Pharmacokinetic Parameters of Lopinavir, Mean ± SD Pharmacokinetic ParameterTwice DailyaOnce DailybCmax(mcg/mL) 9.8 ± 3.7 11.8 ± 3.7 Cmin(mcg/mL) 5.5 ± 2.7 1.7 ± 1.6 AUCtau(mcg•h/mL) 92.6 ± 36.7 154.1 ± 61.4 a. 19 HIV-1 subjects, lopinavir and ritonavir 400/100 mg twice daily
b. 24 HIV-1 subjects, lopinavir and ritonavir 800/200 mg + emtricitabine 200 mg + tenofovir DF 300 mgSpecific PopulationsGender, Race and AgeNo gender or race related pharmacokinetic differences have been observed in adult patients. Lopinavir pharmacokinetics have not been studied in elderly patients.
Pediatric PatientsThe 230/57.5 mg/m2twice daily regimen without nevirapine and the 300/75 mg/m2twice daily regimen with nevirapine provided lopinavir plasma concentrations similar to those obtained in adult patients receiving the 400/100 mg twice daily regimen without nevirapine.
Table 15. Lopinavir Pharmacokinetic Data from Pediatric Clinical Trials, Mean ± SD Cmax(mcg/mL)Cmin(mcg/mL)AUC12(mcg•hr/m)Age ≥ 14 Days to < 6 Weeks Cohort (N = 9):5.17 ± 1.84a 1.40 ± 0.48a 43.39 ± 14.80a Age ≥ 6 Weeks to < 6 Months Cohort (N = 18):9.39 ± 4.91a 1.95 ± 1.80a 74.50 ± 37.87a Age ≥ 6 Months to ≤ 12 years Cohort (N = 24):8.2 ± 2.9b 3.4 ± 2.1b 72.6 ± 31.1b 10.0 ± 3.3c 3.6 ± 3.5c 85.8 ± 36.9c a. Lopinavir and ritonavir oral solution 300/75 mg/m2twice daily without concomitant NNRTI therapy
b. Lopinavir and ritonavir oral solution 230/57.5 mg/m2twice daily without nevirapine (n=12)
c. Lopinavir and ritonavir oral solution 300/75 mg/m2twice daily with nevirapine (n=12)PregnancyThe C12hvalues of lopinavir were lower during the second and third trimester by approximately 40% as compared to post-partum in 12 HIV-infected pregnant women received lopinavir and ritonavir 400 mg/100 mg twice daily. Yet this decrease is not considered clinically relevant in patients with no documented lopinavir and ritonavir-associated resistance substitutions receiving 400 mg/100 mg twice daily
[see Use in Specific Populations (8.1)].Renal ImpairmentLopinavir pharmacokinetics have not been studied in patients with renal impairment; however, since the renal clearance of lopinavir is negligible, a decrease in total body clearance is not expected in patients with renal impairment.
Hepatic ImpairmentMultiple dosing of lopinavir and ritonavir 400/100 mg twice daily to HIV-1 and HCV co-infected patients with mild to moderate hepatic impairment (n = 12) resulted in a 30% increase in lopinavir AUC and 20% increase in Cmaxcompared to HIV-1 infected subjects with normal hepatic function (n = 12). Additionally, the plasma protein binding of lopinavir was statistically significantly lower in both mild and moderate hepatic impairment compared to controls (99.09 vs. 99.31%, respectively). Lopinavir and ritonavir has not been studied in patients with severe hepatic impairment
[see Warnings and Precautions (5.4)andUse in Specific Populations (8.6)].Drug InteractionsLopinavir and ritonavir is an inhibitor of the P450 isoform CYP3A
in vitro. Lopinavir and ritonavir does not inhibit CYP2D6, CYP2C9, CYP2C19, CYP2E1, CYP2B6 or CYP1A2 at clinically relevant concentrations.Lopinavir and ritonavir has been shown
in vivoto induce its own metabolism and to increase the biotransformation of some drugs metabolized by cytochrome P450 enzymes and by glucuronidation.The effects of co-administration of lopinavir and ritonavir on the AUC, Cmaxand Cminare summarized in Table 16 (effect of other drugs on lopinavir) and Table 17 (effect of lopinavir and ritonavir on other drugs). For information regarding clinical recommendations, see Table 12 in Drug Interactions (7).
Table 16. Drug Interactions: Pharmacokinetic Parameters for Lopinavir in the Presence of the Co-administered Drug for Recommended Alterations in Dose or Regimen Co- administered DrugDose of Co- administered Drug(mg)Dose ofLopinavir and Ritonavir(mg)nRatio (in combination with Co-administered drug/alone) of Lopinavir Pharmacokinetic Parameters (90% CI);No Effect = 1.00CmaxAUCCminEfavirenz1 600 at bedtime 400/100 capsule twice daily 11, 73 0.97
(0.78, 1.22)0.81
(0.64, 1.03)0.61
(0.38, 0.97)600 at bedtime 500/125 tablet twice daily 19 1.12
(1.02, 1.23)1.06
(0.96, 1.17)0.90
(0.78, 1.04)600 at bedtime 600/150 tablet twice daily 23 1.36
(1.28, 1.44)1.36
(1.28, 1.44)1.32
(1.21, 1.44)Etravirine 200 twice daily 400/100 mg twice daily (tablets) 16 0.89
(0.82 to 0.96)0.87
(0.83 to 0.92)0.80
(0.73 to 0.88)Fosamprenavir2 700 twice daily plus ritonavir 100 twice daily 400/100 capsule twice daily 18 1.30
(0.85, 1.47)1.37
(0.80, 1.55)1.52
(0.72, 1.82)Ketoconazole 200 single dose 400/100 capsule twice daily 12 0.89
(0.80, 0.99)0.87
(0.75, 1.00)0.75
(0.55, 1.00)Nelfinavir 1,000 twice daily 400/100 capsule twice daily 13 0.79
(0.70, 0.89)0.73
(0.63, 0.85)0.62
(0.49, 0.78)Nevirapine 200 twice daily steady-state 400/100 capsule twice daily 22, 193 0.81
(0.62, 1.05)0.73
(0.53, 0.98)0.49
(0.28, 0.74)7 mg/kg or 4 mg/kg once daily; twice daily 1 wk5 (> 1 yr) 300/ 75 mg/m2oral solution twice daily 12, 153 0.86
(0.64, 1.16)0.78
(0.56, 1.09)0.45
(0.25, 0.81)Ombitasvir/ paritaprevir/ ritonavir+ dasabuvir2 25/150/100 +
dasabuvir 400400/100 tablet twice daily 6 0.87
(0.76, 0.99)0.94
(0.81, 1.10)1.15
(0.93, 1.42)Omeprazole 40 once daily, 5 d 400/100 tablet twice daily, 10 d 12 1.08
(0.99, 1.17)1.07
(0.99, 1.15)1.03
(0.90, 1.18)40 once daily, 5 d 800/200 tablet once daily, 10 d 12 0.94
(0.88, 1.00)0.92
(0.86, 0.99)0.71
(0.57, 0.89)Pravastatin 20 once daily, 4 d 400/100 capsule twice daily, 14 d 12 0.98
(0.89, 1.08)0.95
(0.85, 1.05)0.88
(0.77, 1.02)Ranitidine 150 single dose 400/100 tablet twice daily, 10 d 12 0.99
(0.95, 1.03)0.97
(0.93, 1.01)0.90
(0.85, 0.95)150 single dose 800/200 tablet once daily, 10 d 10 0.97
(0.95, 1.00)0.95
(0.91, 0.99)0.82
(0.74, 0.91)Rifabutin 150 once daily 400/100 capsule twice daily 14 1.08
(0.97, 1.19)1.17
(1.04, 1.31)1.20
(0.96, 1.65)Rifampin 600 once daily 400/100 capsule twice daily 22 0.45
(0.40, 0.51)0.25
(0.21, 0.29)0.01
(0.01, 0.02)600 once daily 800/200 capsule twice daily 10 1.02
(0.85, 1.23)0.84
(0.64, 1.10)0.43
(0.19, 0.96)600 once daily 400/400 capsule twice daily 9 0.93
(0.81, 1.07)0.98
(0.81, 1.17)1.03
(0.68, 1.56)Rilpivirine 150 once daily 400/100 twice daily (capsules) 15 0.96
(0.88 to 1.05)0.99
(0.89 to 1.10)0.89
(0.73 to 1.08)Ritonavir 100 twice daily 400/100 capsule twice daily 8, 213 1.28
(0.94, 1.76)1.46
(1.04, 2.06)2.16
(1.29, 3.62)Tipranavir/ ritonavir 500/200 twice daily 400/100 capsule twice daily 21, 693 0.53
(0.40, 0.69)0.45
(0.32, 0.63)0.30
(0.17, 0.51)
0.484
(0.40, 0.58)1.000000000000000e+00Reference for comparison is lopinavir/ritonavir 400/100 mg twice daily without efavirenz.
2.000000000000000e+00Data extracted from the U.S. prescribing information of co-administered drugs.
3.000000000000000e+00Parallel group design
4.000000000000000e+00Drug levels obtained at 8 to 16 hours post dose
N/A = Not available.Table 17. Drug Interactions: Pharmacokinetic Parameters for Co-administered Drug in the Presence of Lopinavir and Ritonavir for Recommended Alterations in Dose or Regimen Co- administered DrugDose of Co- administered Drug (mg)Dose ofLopinavir and Ritonavir(mg)nRatio (in combination withLopinavir and Ritonavir/alone) of Co-administered Drug Pharmacokinetic Parameters (90% CI); No Effect = 1.00CmaxAUCCminBedaquiline1 400 single dose 400/100 twice daily N/A N/A 1.22
(1.11, 1.34)N/A Efavirenz 600 at bedtime 400/100
capsule twice daily11, 123 0.91
(0.72, 1.15)0.84
(0.62, 1.15)0.84
(0.58, 1.20)Elbasvir/ grazoprevir1 50 once daily 400/100 twice daily 10 2.87
(2.29, 3.58)3.71
(3.05, 4.53)4.58
(3.72, 5.64)200 once daily 13 7.31
(5.65, 9.45)12.86
(10.25, 16.13)21.70
(12.99, 36.25)Ethinyl Estradiol 35 mcg once daily
(Ortho Novum®)400/100
capsule twice daily12 0.59
(0.52, 0.66)0.58
(0.54, 0.62)0.42
(0.36, 0.49)Etravirine 200 twice daily 400/100 tablet twice day 16 0.70
(0.64 to 0.78)0.65
(0.59 to 0.71)0.55
(0.49 to 0.62)Fosamprenavir1 700 twice daily plus ritonavir 100 twice daily 400/100
capsule twice daily18 0.42
(0.30, 0.58)0.37
(0.28, 0.49)0.35
(0.27, 0.46)Indinavir 600 twice daily combo nonfasting vs. 800 three times daily alone fasting 400/100
capsule twice daily13 0.71
(0.63, 0.81)0.91
(0.75, 1.10)3.47
(2.60, 4.64)Ketoconazole 200 single dose 400/100
capsule twice daily12 1.13
(0.91, 1.40)3.04
(2.44, 3.79)N/A Maraviroc1 300 twice daily 400/100
twice daily11 1.97
(1.66, 2.34)3.95
(3.43, 4.56)9.24
(7.98, 10.7)Methadone 5 single dose 400/100
capsule twice daily11 0.55
(0.48, 0.64)0.47
(0.42, 0.53)N/A Nelfinavir 1,000 twice daily combo vs.
1,250 twice daily alone400/100
capsule twice daily13 0.93
(0.82, 1.05)1.07
(0.95, 1.19)1.86
(1.57, 2.22)M8 metabolite 2.36
(1.91, 2.91)3.46
(2.78, 4.31)7.49
(5.85, 9.58)Nevirapine 200 once daily twice daily 400/100
capsule twice daily5, 63 1.05
(0.72, 1.52)1.08
(0.72, 1.64)1.15
(0.71, 1.86)Norethindrone 1 once daily
(Ortho Novum®)400/100
capsule twice daily12 0.84
(0.75, 0.94)0.83
(0.73, 0.94)0.68
(0.54, 0.85)Ombitasvir/ paritaprevir/ ritonavir+ dasabuvir1 25/150/100 +
dasabuvir 400400/100
tablet twice
daily6 1.14
(1.01, 1.28)1.17
(1.07, 1.28)1.24
(1.14, 1.34)2.04
(1.30, 3.20)2.17
(1.63, 2.89)2.36
(1.00, 5.55)1.55
(1.16, 2.09)2.05
(1.49, 2.81)5.25
(3.33, 8.28)0.99
(0.75, 1.31)0.93
(0.75, 1.15)0.68
(0.57, 0.80)Pitavastatin1 4 once daily 400/100 tablet twice daily 23 0.96
(0.84 to 1.10)0.80
(0.73 to 0.87)N/A Pravastatin 20 once daily 400/100 capsule twice daily 12 1.26
(0.87, 1.83)1.33
(0.91, 1.94)N/A Rifabutin 150 once daily
combo vs. 300
once daily
alone400/100 capsule twice daily 12 2.12
(1.89, 2.38)3.03
(2.79, 3.30)4.90
(3.18, 5.76)25- O-desacetyl rifabutin23.6
(13.7, 25.3)47.5
(29.3, 51.8)94.9
(74.0, 122)Rifabutin + 25- O-desacetyl rifabutin3.46
(3.07, 3.91)5.73
(5.08, 6.46)9.53
(7.56, 12.01)Rilpivirine 150 once daily 400/100 capsules twice daily 15 1.29
(1.18 to 1.40)1.52
(1.36 to 1.70)1.74
(1.46 to 2.08)Rosuvastatin2 20 once daily 400/100 tablet twice daily 15 4.66
(3.4, 6.4)2.08
(1.66, 2.6)1.04
(0.9, 1.2)Tenofovir alafenamide1 10 once daily 800/200
tablet once daily10 2.19
(1.72, 2.79)1.47
(1.17, 1.85)N/A Tenofovir disoproxil fumarate1 300 once daily 400/100 capsule twice daily 24 No Change 1.32
(1.26, 1.38)1.51
(1.32, 1.66)1.000000000000000e+00Data extracted from the U.S. prescribing information of co-administered drugs.
2.000000000000000e+00Kiser, et al. J Acquir Immune Defic Syndr. 2008 Apr 15; 47(5):570-8.
3Parallel group design
N/A = Not available.
○ Anticancer Agents: apalutamide
○ Antimycobacterial: rifampin
○ Herbal Products: St. John's Wort (hypericum perforatum)