Lorazepam Prescribing Information
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (seeand
WARNINGSRisks from Concomitant Use with OpioidsConcomitant use of benzodiazepines, including lorazepam tablets, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe lorazepam tablets concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of lorazepam tablets than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking lorazepam tablets, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when lorazepam tablets are used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see
PRECAUTIONS: Drug Interactions).Abuse, Misuse, and AddictionThe use of benzodiazepines, including lorazepam tablets, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see
DRUG ABUSE AND DEPENDENCE: Abuse).Before prescribing lorazepam tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of lorazepam tablets, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of lorazepam tablets along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal ReactionsTo reduce the risk of withdrawal reactions, use a gradual taper to discontinue lorazepam tablets or reduce the dosage (a patient-specific plan should be used to taper the dose) (see
DOSAGE AND ADMINSTRATION: Discontinuation or Dosage Reduction of Lorazepam Tablets).Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal ReactionsThe continued use of benzodiazepines, including lorazepam tablets, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of lorazepam tablets after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see
DRUG ABUSE AND DEPENDENCE: Dependence).Protracted Withdrawal SyndromeIn some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see
DRUG ABUSE AND DEPENDENCE: Dependence).Pre-existing depression may emerge or worsen during use of benzodiazepines including lorazepam. Lorazepam tablets are not recommended for use in patients with a primary depressive disorder or psychosis.
Use of benzodiazepines, including lorazepam, both used alone and in combination with other CNS depressants, may lead to potentially fatal respiratory depression (see
PRECAUTIONS: Drug Interactions).As with all patients on CNS-depressant drugs, patients receiving lorazepam should be warned not to operate dangerous machinery or motor vehicles and that their tolerance for alcohol and other CNS depressants will be diminished.
Neonatal Sedation and Withdrawal SyndromeUse of lorazepam tablets late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (see
PRECAUTIONS: Pregnancy). Monitor neonates exposed to lorazepam tablets during pregnancy or labor for signs of sedation and monitor neonates exposed to lorazepam tablets during pregnancy for signs of withdrawal; manage these neonates accordingly.).PRECAUTIONSIn patients with depression, a possibility for suicide should be borne in mind; benzodiazepines should not be used in such patients without adequate antidepressant therapy.
Lorazepam should be used with caution in patients with compromised respiratory function (e.g., COPD, sleep apnea syndrome).
Elderly or debilitated patients may be more susceptible to the sedative effects of lorazepam. Therefore, these patients should be monitored frequently and have their dosage adjusted carefully according to patient response; the initial dosage should not exceed 2 mg.
Paradoxical reactions have been occasionally reported during benzodiazepine use. Such reactions may be more likely to occur in children and the elderly. Should these occur, use of the drug should be discontinued.
The usual precautions for treating patients with impaired renal or hepatic function should be observed. As with all benzodiazepines, the use of lorazepam may worsen hepatic encephalopathy; therefore, lorazepam should be used with caution in patients with severe hepatic insufficiency and/or encephalopathy. Dosage for patients with severe hepatic insufficiency should be adjusted carefully according to patient response; lower doses may be sufficient in such patients.
In patients where gastrointestinal or cardiovascular disorders coexist with anxiety, it should be noted that lorazepam has not been shown to be of significant benefit in treating the gastrointestinal or cardiovascular component.
Esophageal dilation occurred in rats treated with lorazepam for more than 1 year at 6 mg/kg/day. The no-effect dose was 1.25 mg/kg/day (approximately 6 times the maximum human therapeutic dose of 10 mg/day). The effect was reversible only when the treatment was withdrawn within 2 months of first observation of the phenomenon. The clinical significance of this is unknown. However, use of lorazepam for prolonged periods and in geriatric patients requires caution, and there should be frequent monitoring for symptoms of upper GI disease.
Safety and effectiveness of lorazepam tablets in children of less than 12 years have not been established.
Information for PatientsAdvise the patient to read the FDA-approved patient labeling (Medication Guide).
Risks from Concomitant Use with OpioidsAdvise both patients and caregivers about the risks of potentially fatal respiratory depression and sedation when lorazepam tablets are used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see
WARNINGS: Risks from Concomitant Use of Opioidsand PRECAUTIONS: Drug Interactions).Abuse, Misuse, and AddictionInform patients that the use of lorazepam tablets even at recommended doses, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug (see
WARNINGS: Abuse Misuse, and AddictionandDRUG ABUSE AND DEPENDENCE).Withdrawal ReactionsInform patients that the continued use of lorazepam tablets may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of lorazepam tablets may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of lorazepam tablets may require a slow taper (see
WARNINGS: Dependence and Withdrawal ReactionsandDRUG ABUSE AND DEPENDENCE).PregnancyAdvise pregnant females that use of lorazepam tablets late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns (see
WARNINGS: Neonatal Sedation and Withdrawal SyndromeandPRECAUTIONS: Pregnancy). Instruct patients to inform their healthcare provider if they are pregnant.Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to lorazepam tablets during pregnancy (see
PRECAUTIONS: Pregnancy).NursingInstruct patients to notify their healthcare provider if they are breastfeeding or intend to breastfeed. Instruct breastfeeding patients using lorazepam tablets to monitor infants for excessive sedation, poor feeding and poor weight gain, and to seek medical attention if they notice these signs (see
PRECAUTIONS: Nursing Mothers).Essential Laboratory TestsSome patients on lorazepam tablets have developed leukopenia, and some have had elevations of LDH. As with other benzodiazepines, periodic blood counts and liver function tests are recommended for patients on long-term therapy.
Drug InteractionsThe concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAAsites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists.
Limit dosage and duration of concomitant use of benzodiazepines and opioids, and monitor patients closely for respiratory depression and sedation.
The benzodiazepines, including lorazepam tablets, produce increased CNS-depressant effects when administered with other CNS depressants such as alcohol, barbiturates, antipsychotics, sedative/hypnotics, anxiolytics, antidepressants, narcotic analgesics, sedative antihistamines, anticonvulsants, and anesthetics.
Concomitant use of clozapine and lorazepam may produce marked sedation, excessive salivation, hypotension, ataxia, delirium, and respiratory arrest.
Concurrent administration of lorazepam with valproate results in increased plasma concentrations and reduced clearance of lorazepam. Lorazepam dosage should be reduced to approximately 50% when coadministered with valproate.
Concurrent administration of lorazepam with probenecid may result in a more rapid onset or prolonged effect of lorazepam due to increased half-life and decreased total clearance. Lorazepam dosage needs to be reduced by approximately 50% when coadministered with probenecid.
The effects of probenecid and valproate on lorazepam may be due to inhibition of glucuronidation.
Administration of theophylline or aminophylline may reduce the sedative effects of benzodiazepines, including lorazepam.
Carcinogenesis and MutagenesisNo evidence of carcinogenic potential emerged in rats during an 18-month study with lorazepam tablets. No studies regarding mutagenesis have been performed.
PregnancyPregnancy Exposure RegistryThere is a pregnancy registry that monitors pregnancy outcomes in women exposed to psychiatric medications, including Ativan, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at
https://womensmentalhealth.org/pregnancyregistry/.Risk SummaryNeonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal (see
WARNINGS: Neonatal Sedation and Withdrawal SyndromeandClinical Considerations). Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects(see Data).The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical ConsiderationsFetal/Neonatal Adverse ReactionsBenzodiazepines cross the placenta and may produce respiratory depression, hypotonia and sedation in neonates. Monitor neonates exposed to Ativan during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to lorazepam tablets during pregnancy for signs of withdrawal. Manage these neonates accordingly (see
WARNINGS: Neonatal Sedation and Withdrawal Syndrome).DataHuman DataPublished data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Animal DataReproductive studies in animals were performed in mice, rats, and two strains of rabbits. Occasional anomalies (reduction of tarsals, tibia, metatarsals, malrotated limbs, gastroschisis, malformed skull, and microphthalmia) were seen in drug-treated rabbits without relationship to dosage. Although all of these anomalies were not present in the concurrent control group, they have been reported to occur randomly in historical controls. At doses of 40 mg/kg and higher, there was evidence of fetal resorption and increased fetal loss in rabbits which was not seen at lower doses.
Nursing MothersRisk SummaryLorazepam is present in breast milk. There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. The effects of lorazepam on milk production are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for lorazepam tablets and any potential adverse effects on the breastfed infant from lorazepam tablets or from the underlying maternal condition.
Clinical ConsiderationsInfants exposed to lorazepam tablets through breast milk should be monitored for sedation, poor feeding and poor weight gain.
Geriatric UseClinical studies of lorazepam tablets generally were not adequate to determine whether subjects aged 65 and over respond differently than younger subjects; however, the incidence of sedation and unsteadiness was observed to increase with age (see
ADVERSE REACTIONS).Age does not appear to have a significant effect on lorazepam kinetics (see
CLINICAL PHARMACOLOGY).Clinical circumstances, some of which may be more common in the elderly, such as hepatic or renal impairment, should be considered. Greater sensitivity (e.g., sedation) of some older individuals cannot be ruled out. In general, dose selection for an elderly patient should be cautious, and lower doses may be sufficient in these patients (see
DOSAGE AND ADMINISTRATION).
- The use of benzodiazepines, including lorazepam tablets, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing lorazepam tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (see).
WARNINGSRisks from Concomitant Use with OpioidsConcomitant use of benzodiazepines, including lorazepam tablets, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe lorazepam tablets concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of lorazepam tablets than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking lorazepam tablets, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when lorazepam tablets are used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see
PRECAUTIONS: Drug Interactions).Abuse, Misuse, and AddictionThe use of benzodiazepines, including lorazepam tablets, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see
DRUG ABUSE AND DEPENDENCE: Abuse).Before prescribing lorazepam tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of lorazepam tablets, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of lorazepam tablets along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal ReactionsTo reduce the risk of withdrawal reactions, use a gradual taper to discontinue lorazepam tablets or reduce the dosage (a patient-specific plan should be used to taper the dose) (see
DOSAGE AND ADMINSTRATION: Discontinuation or Dosage Reduction of Lorazepam Tablets).Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal ReactionsThe continued use of benzodiazepines, including lorazepam tablets, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of lorazepam tablets after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see
DRUG ABUSE AND DEPENDENCE: Dependence).Protracted Withdrawal SyndromeIn some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see
DRUG ABUSE AND DEPENDENCE: Dependence).Pre-existing depression may emerge or worsen during use of benzodiazepines including lorazepam. Lorazepam tablets are not recommended for use in patients with a primary depressive disorder or psychosis.
Use of benzodiazepines, including lorazepam, both used alone and in combination with other CNS depressants, may lead to potentially fatal respiratory depression (see
PRECAUTIONS: Drug Interactions).As with all patients on CNS-depressant drugs, patients receiving lorazepam should be warned not to operate dangerous machinery or motor vehicles and that their tolerance for alcohol and other CNS depressants will be diminished.
Neonatal Sedation and Withdrawal SyndromeUse of lorazepam tablets late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (see
PRECAUTIONS: Pregnancy). Monitor neonates exposed to lorazepam tablets during pregnancy or labor for signs of sedation and monitor neonates exposed to lorazepam tablets during pregnancy for signs of withdrawal; manage these neonates accordingly. - The continued use of benzodiazepines, including lorazepam tablets, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of lorazepam tablets after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue lorazepam tablets or reduce the dosage (and
DOSAGE AND ADMINISTRATIONLorazepam tablets are administered orally. For optimal results, dose, frequency of administration, and duration of therapy should be individualized according to patient response. To facilitate this, 0.5 mg, 1 mg, and 2 mg tablets are available.
The usual range is 2 to 6 mg/day given in divided doses, the largest dose being taken before bedtime, but the daily dosage may vary from 1 to 10 mg/day.
For anxiety, most patients require an initial dose of 2 to 3 mg/day given two times a day or three times a day.
For insomnia due to anxiety or transient situational stress, a single daily dose of 2 to 4 mg may be given, usually at bedtime.
For elderly or debilitated patients, an initial dosage of 1 to 2 mg/day in divided doses is recommended, to be adjusted as needed and tolerated.
The dosage of lorazepam tablets should be increased gradually when needed to help avoid adverse effects. When higher dosage is indicated, the evening dose should be increased before the daytime doses.
Discontinuation or Dosage Reduction of Lorazepam TabletsTo reduce the risk of withdrawal reactions, use a gradual taper to discontinue lorazepam tablets or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see
WARNINGS: Dependence and Withdrawal ReactionsandDRUG ABUSE AND DEPENDENCE: Dependence).).WARNINGSRisks from Concomitant Use with OpioidsConcomitant use of benzodiazepines, including lorazepam tablets, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe lorazepam tablets concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of lorazepam tablets than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking lorazepam tablets, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when lorazepam tablets are used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see
PRECAUTIONS: Drug Interactions).Abuse, Misuse, and AddictionThe use of benzodiazepines, including lorazepam tablets, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see
DRUG ABUSE AND DEPENDENCE: Abuse).Before prescribing lorazepam tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of lorazepam tablets, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of lorazepam tablets along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal ReactionsTo reduce the risk of withdrawal reactions, use a gradual taper to discontinue lorazepam tablets or reduce the dosage (a patient-specific plan should be used to taper the dose) (see
DOSAGE AND ADMINSTRATION: Discontinuation or Dosage Reduction of Lorazepam Tablets).Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal ReactionsThe continued use of benzodiazepines, including lorazepam tablets, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of lorazepam tablets after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see
DRUG ABUSE AND DEPENDENCE: Dependence).Protracted Withdrawal SyndromeIn some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see
DRUG ABUSE AND DEPENDENCE: Dependence).Pre-existing depression may emerge or worsen during use of benzodiazepines including lorazepam. Lorazepam tablets are not recommended for use in patients with a primary depressive disorder or psychosis.
Use of benzodiazepines, including lorazepam, both used alone and in combination with other CNS depressants, may lead to potentially fatal respiratory depression (see
PRECAUTIONS: Drug Interactions).As with all patients on CNS-depressant drugs, patients receiving lorazepam should be warned not to operate dangerous machinery or motor vehicles and that their tolerance for alcohol and other CNS depressants will be diminished.
Neonatal Sedation and Withdrawal SyndromeUse of lorazepam tablets late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (see
PRECAUTIONS: Pregnancy). Monitor neonates exposed to lorazepam tablets during pregnancy or labor for signs of sedation and monitor neonates exposed to lorazepam tablets during pregnancy for signs of withdrawal; manage these neonates accordingly.
Lorazepam tablets are indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety or anxiety associated with depressive symptoms. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The effectiveness of lorazepam tablets in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
Lorazepam tablets are administered orally. For optimal results, dose, frequency of administration, and duration of therapy should be individualized according to patient response. To facilitate this, 0.5 mg, 1 mg, and 2 mg tablets are available.
The usual range is 2 to 6 mg/day given in divided doses, the largest dose being taken before bedtime, but the daily dosage may vary from 1 to 10 mg/day.
For anxiety, most patients require an initial dose of 2 to 3 mg/day given two times a day or three times a day.
For insomnia due to anxiety or transient situational stress, a single daily dose of 2 to 4 mg may be given, usually at bedtime.
For elderly or debilitated patients, an initial dosage of 1 to 2 mg/day in divided doses is recommended, to be adjusted as needed and tolerated.
The dosage of lorazepam tablets should be increased gradually when needed to help avoid adverse effects. When higher dosage is indicated, the evening dose should be increased before the daytime doses.
Lorazepam tablets are contraindicated in patients with:
- hypersensitivity to benzodiazepines or to any components of the formulation.
- acute narrow-angle glaucoma.
Most adverse reactions to benzodiazepines, including CNS effects and respiratory depression, are dose dependent, with more severe effects occurring with high doses.
In a sample of about 3500 patients treated for anxiety, the most frequent adverse reaction to lorazepam tablets was sedation (15.9%), followed by dizziness (6.9%), weakness (4.2%), and unsteadiness (3.4%). The incidence of sedation and unsteadiness increased with age.
Other adverse reactions to benzodiazepines, including lorazepam are fatigue, drowsiness, amnesia, memory impairment, confusion, disorientation, depression, unmasking of depression, disinhibition, euphoria, suicidal ideation/attempt, ataxia, asthenia, extrapyramidal symptoms, convulsions/seizures, tremor, vertigo, eye function/visual disturbance (including diplopia and blurred vision), dysarthria/slurred speech, change in libido, impotence, decreased orgasm; headache, coma; respiratory depression, apnea, worsening of sleep apnea, worsening of obstructive pulmonary disease; gastrointestinal symptoms including nausea, change in appetite, constipation, jaundice, increase in bilirubin, increase in liver transaminases, increase in alkaline phosphatase; hypersensitivity reactions, anaphylactoid reactions; dermatological symptoms, allergic skin reactions, alopecia; syndrome of inappropriate antidiuretic hormone (SIADH), hyponatremia; thrombocytopenia, agranulocytosis, pancytopenia; hypothermia; and autonomic manifestations.
Paradoxical reactions, including anxiety, excitation, agitation, hostility, aggression, rage, sleep disturbances/insomnia, sexual arousal, and hallucinations may occur. Small decreases in blood pressure and hypotension may occur but are usually not clinically significant, probably being related to the relief of anxiety produced by lorazepam tablets.
Lorazepam Tablets, USP, an antianxiety agent, has the chemical formula, 7-Chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-2
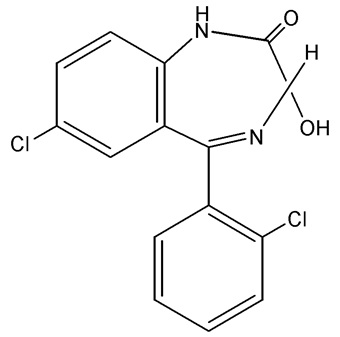
C
15H
10Cl
2N
2O
2 MW: 321.16
It is a white or almost white crystalline powder almost insoluble in water. Each Lorazepam Tablet, USP, to be taken orally, contains 0.5 mg, 1 mg, or 2 mg of Lorazepam, USP. The inactive ingredients present are croscarmellose sodium, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate and tribasic calcium phosphate.