Lorazepam Prescribing Information
•
NOTE: CONTAINS BENZYL ALCOHOL (see
EQUIPMENT NECESSARY TO MAINTAIN A PATENT AIRWAY SHOULD BE IMMEDIATELY AVAILABLE PRIOR TO INTRAVENOUS ADMINISTRATION OF LORAZEPAM (see
Lorazepam Injection is contraindicated in patients with a known sensitivity to benzodiazepines or its vehicle (polyethylene glycol, propylene glycol and benzyl alcohol), in patients with acute narrow-angle glaucoma, or in patients with sleep apnea syndrome. It is also contraindicated in patients with severe respiratory insufficiency, except in those patients requiring relief of anxiety and/or diminished recall of events while being mechanically ventilated. The use of Lorazepam Injection intra-arterially is contraindicated because, as with other injectable benzodiazepines, inadvertent intra-arterial injection may produce arteriospasm resulting in gangrene which may require amputation (see
Lorazepam Injection is contraindicated for use in premature infants because the formulation contains benzyl alcohol. (See
INTERACTION WITH BENZODIAZEPINES AND OTHER CNS DEPRESSANTS
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Monitor patients closely for respiratory depression and sedation.
Lorazepam Injection, like other injectable benzodiazepines, produces additive depression of the central nervous system when administered with other CNS depressants such as ethyl alcohol, phenothiazines, barbiturates, MAO inhibitors, and other antidepressants.
When scopolamine is used concomitantly with injectable lorazepam, an increased incidence of sedation, hallucinations and irrational behavior has been observed.
There have been rare reports of significant respiratory depression, stupor and/or hypotension with the concomitant use of loxapine and lorazepam.
Marked sedation, excessive salivation, ataxia, and, rarely, death have been reported with the concomitant use of clozapine and lorazepam.
Apnea, coma, bradycardia, arrhythmias, heart arrest, and death have been reported with the concomitant use of haloperidol and lorazepam.
The risk of using lorazepam in combination with scopolamine, loxapine, clozapine, haloperidol, or other CNS-depressant drugs has not been systematically evaluated. Therefore, caution is advised if the concomitant administration of lorazepam and these drugs is required.
Concurrent administration of any of the following drugs with lorazepam had no effect on the pharmacokinetics of lorazepam: metoprolol, cimetidine, ranitidine, disulfiram, propranolol, metronidazole, and propoxyphene. No change in lorazepam dosage is necessary when concomitantly given with any of these drugs.
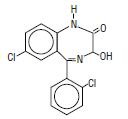
Lorazepam, a benzodiazepine with antianxiety, sedative, and anticonvulsant effects, is intended for the intramuscular or intravenous routes of administration. It has the chemical formula: 7-chloro-5(2-chlorophenyl)-1,3-dihydro-3-hydroxy-2
Lorazepam interacts with the γ-aminobutyric acid (GABA)-benzodiazepine receptor complex, which is widespread in the brain of humans as well as other species. This interaction is presumed to be responsible for lorazepam’s mechanism of action. Lorazepam exhibits relatively high and specific affinity for its recognition site but does not displace GABA. Attachment to the specific binding site enhances the affinity of GABA for its receptor site on the same receptor complex. The pharmacodynamic consequences of benzodiazepine agonist actions include antianxiety effects, sedation, and reduction of seizure activity. The intensity of action is directly related to the degree of benzodiazepine receptor occupancy.