Lumason Prescribing Information
fatalities, have occurred uncommonly during or following the injection
of ultrasound contrast agents, including sulfur hexafluoride lipid
microspheres
Reactions
Serious cardiopulmonary reactions, including fatalities have occurred
uncommonly during or shortly following administration of ultrasound
contrast agents, including Lumason. These reactions typically occurred
within 30 minutes of administration. The risk for these reactions
may be increased among patients with unstable cardiopulmonary conditions
(acute myocardial infarction, acute coronary artery syndromes, worsening
or unstable congestive heart failure, or serious ventricular arrhythmias).
Always have cardiopulmonary resuscitation personnel and equipment
readily available prior to Lumason administration and monitor all
patients for acute reactions.
The reported reactions that may follow the
administration of ultrasound contrast agents include: fatal cardiac
or respiratory arrest, shock, syncope, symptomatic arrhythmias (atrial
fibrillation, tachycardia, bradycardia, supraventricular tachycardia,
ventricular fibrillation, and ventricular tachycardia), hypertension,
hypotension, dyspnea, hypoxia, chest pain, respiratory distress, stridor,
wheezing, loss of consciousness, and convulsions.
within 30 minutes of administration
(
Reactions
Serious cardiopulmonary reactions, including fatalities have occurred
uncommonly during or shortly following administration of ultrasound
contrast agents, including Lumason. These reactions typically occurred
within 30 minutes of administration. The risk for these reactions
may be increased among patients with unstable cardiopulmonary conditions
(acute myocardial infarction, acute coronary artery syndromes, worsening
or unstable congestive heart failure, or serious ventricular arrhythmias).
Always have cardiopulmonary resuscitation personnel and equipment
readily available prior to Lumason administration and monitor all
patients for acute reactions.
The reported reactions that may follow the
administration of ultrasound contrast agents include: fatal cardiac
or respiratory arrest, shock, syncope, symptomatic arrhythmias (atrial
fibrillation, tachycardia, bradycardia, supraventricular tachycardia,
ventricular fibrillation, and ventricular tachycardia), hypertension,
hypotension, dyspnea, hypoxia, chest pain, respiratory distress, stridor,
wheezing, loss of consciousness, and convulsions.
- Assess all patients for the presence of any condition
that precludes administration[see Contraindications (.)]4 CONTRAINDICATIONSLumason is contraindicated in patients with known or suspected:- Hypersensitivity to sulfur hexafluoride lipid microsphere or its components, such as polyethylene glycol (PEG)[see Warnings and Precautions and Description ].
- Hypersensitivity to sulfur hexafluoride lipid microspheres
or its components, such as polyethylene glycol (PEG)
- Hypersensitivity to sulfur hexafluoride lipid microsphere or its components, such as polyethylene glycol (PEG)
- Always have resuscitation equipment and trained personnel
readily available[see Warnings and Precautions (.)]5.1 Serious Cardiopulmonary
ReactionsSerious cardiopulmonary reactions, including fatalities have occurred
uncommonly during or shortly following administration of ultrasound
contrast agents, including Lumason. These reactions typically occurred
within 30 minutes of administration. The risk for these reactions
may be increased among patients with unstable cardiopulmonary conditions
(acute myocardial infarction, acute coronary artery syndromes, worsening
or unstable congestive heart failure, or serious ventricular arrhythmias).
Always have cardiopulmonary resuscitation personnel and equipment
readily available prior to Lumason administration and monitor all
patients for acute reactions.The reported reactions that may follow the
administration of ultrasound contrast agents include: fatal cardiac
or respiratory arrest, shock, syncope, symptomatic arrhythmias (atrial
fibrillation, tachycardia, bradycardia, supraventricular tachycardia,
ventricular fibrillation, and ventricular tachycardia), hypertension,
hypotension, dyspnea, hypoxia, chest pain, respiratory distress, stridor,
wheezing, loss of consciousness, and convulsions.
Contraindications (Lumason is contraindicated in patients with known or suspected:
| 4/2021 |
Warnings and Precautions, Hypersensitivity Reactions (Reactions In postmarketing use, serious hypersensitivity reactions were observed during or shortly following sulfur hexafluoride lipid-containing microsphere administration including: Anaphylaxis, with manifestations that may include death, shock, bronchospasm, dyspnea, throat tightness, angioedema, edema (pharyngeal, palatal, mouth, peripheral, localized), swelling (face, eye, lip, tongue, upper airway), facial hypoesthesia, rash, urticaria, pruritus, flushing, and erythema. These reactions may occur in patients with no history of prior exposure to sulfur hexafluoride lipid-containing microspheres. Lumason contains PEG. There may be increased risk of serious reactions including death in patients with prior hypersensitivity reaction(s) to PEG [see Adverse Reactions ] . Clinically assess patients for prior hypersensitivity reactions to products containing PEG, such as certain colonoscopy bowel preparations and laxatives. Always have cardiopulmonary resuscitation personnel and equipment readily available prior to Lumason administration and monitor all patients for hypersensitivity reactions. | 4/2021 |
Lumason is indicated
for use in adult and pediatric patients with suboptimal echocardiograms
to opacify the left ventricular chamber and to improve the delineation
of the left ventricular endocardial border.
Lumason is indicated
for use with ultrasound of the liver in adult and pediatric patients
to characterize focal liver lesions.
Tract
Lumason
is indicated for use in ultrasonography of the urinary tract in pediatric
patients for the evaluation of suspected or known vesicoureteral reflux.
Avoid intra-arterial injection (
Do not administer Lumason by intra-arterial injection
When administering Lumason to
patients with cardiac shunt, microspheres can bypass filtering by
the lung and enter the arterial circulation. Assess patients with
shunts for embolic phenomena following Lumason administration. Lumason
is only for intravenous and/or intravesical administration; do not
administer Lumason by intra-arterial injection
and Administration ].
See Full Prescribing Information for reconstitution instructions (
- Refer to Section 2.3.1 for instructions for using the single patient use kit with diluent provided
- Refer to Section 2.3.2 for instructions for using the 20-vial pack without diluent provided
- Inspect the Lumason kit and its components for signs of damage. Do not use the kit if the protective caps on the Lumason vial and prefilled syringe with 5 mL 0.9% Sodium Chloride Injection, USP are not intact or if the kit shows other signs of damage.
- Under aseptic conditions, reconstitute the Lumason vial using the following illustrated steps:
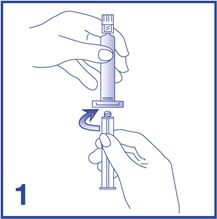
1. Connect the plunger rod to the prefilled 0.9% Sodium Chloride Injection, USP syringe barrel by screwing it clockwise into the syringe (see Figure 1).
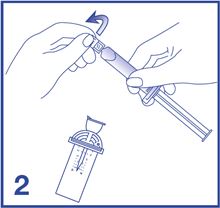
2. Open the Mini-Spike blister and remove the syringe tip cap (see Figure 2).
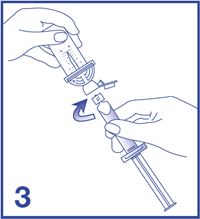
3. Remove the Mini-Spike green cap and connect the syringe to the Mini-Spike by screwing it in clockwise (see Figure 3).
4. Remove the Mini-Spike spike protection and position the spike in the center of the rubber stopper of the vial. Press firmly inward until the spike is fully inserted in the stopper (see Figure 4).
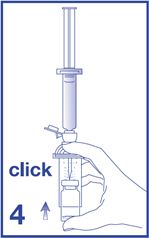
5. Empty the content of the syringe into the vial by pushing on the plunger rod (see Figure 5).
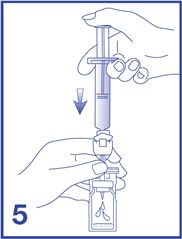
6. Shake vigorously for 20 seconds, mixing all the contents in the vial (see Figure 6). A homogeneous white milky liquid indicates formation of sulfur hexafluoride lipid microspheres.
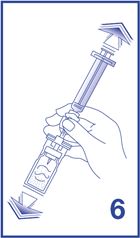
7. For preparation of doses greater than or equal to 1 mL, invert the system and slowly withdraw the intended volume of suspension into the syringe (see Figure 7). For preparation of doses less than 1 mL, withdraw 2 mL of the reconstituted suspension into the 5 mL syringe and measure the volume of Lumason to inject by using the 0.2 mL graduations between the 1 mL and 2 mL marks.
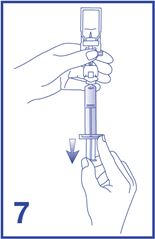
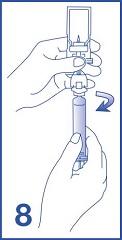
8. Unscrew the syringe from the Mini-Spike (see Figure 8). Peel and remove the diluent label to display the reconstituted product label. For intravenous administration, immediately connect the syringe to a dose administration line (20 G) and administer as directed under the Administration Instructions below. For intravesical administration, immediately connect the syringe to a sterile urinary catheter (6 French to 8 French) and administer as directed under the Administration Instructions below.
- Following reconstitution, Lumason suspension contains 1.5 to 5.6 x108microspheres/mL with 45 mcg/mL of sulfur hexafluoride.
- Use immediately after reconstitution. If the suspension is not used immediately after reconstitution, resuspend the microspheres for a few seconds by hand agitation before the suspension is drawn into the syringe. Reconstituted suspension within a vial may be used for up to 3 hours from the time of its reconstitution. Maintain the vial containing the reconstituted suspension at room temperature 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).










*Please note: This presentation does not include pre-filled syringes of 0.9% Sodium Chloride Injection, USP (Diluent).
Lumason vials are to be used with the supplied Mini-Spike only.
- Inspect the Lumason components for signs of damage. Do not use the Lumason vial if the protective cap on the vial is not intact or other components in the pack show signs of damage.
- Use aseptic conditions for the preparation and administration of Lumason.
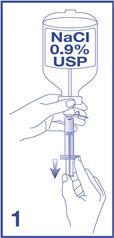
1. Obtain a 5 mL syringe, with luer lock tip, and fill with 5 mL of additive-free 0.9% Sodium Chloride Injection, USP (diluent) (see Figure 1).
- Two healthcare professionals (HCPs) should verify that the solution selected for reconstitution of Lumason is additive-free 0.9% Sodium Chloride Injection, USP.
- Ensure that any air in the syringe is expelled.
[Note: A prefilled syringe containing additive-free 0.9% Sodium Chloride Injection, USP may be used. Ensure that any air in the syringe is expelled.]
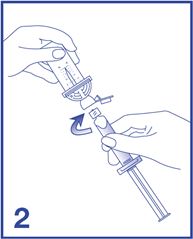
2. Remove the Mini-Spike green cap and connect the syringe to the Mini-Spike by screwing it in clockwise (see Figure 2).
3. Remove the Mini-Spike spike protection and position the spike in the center of the rubber stopper of the vial. Press firmly inward until the spike is fully inserted in the stopper (see Figure 3).

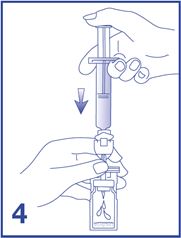
4. Empty the entire 5 mL content of the syringe into the vial by pushing on the plunger rod (see Figure 4).
5. Shake vigorously for 20 seconds, mixing all the contents in the vial (see Figure 5). A homogeneous white milky liquid indicates formation of sulfur hexafluoride lipid microspheres.
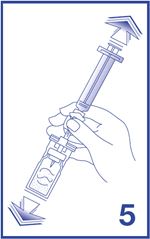
6. To obtain required dose, invert the system and slowly withdraw the intended volume of suspension into the syringe (see Figure 6).
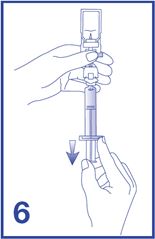
7. Unscrew the syringe from the Mini-Spike (see Figure 7).
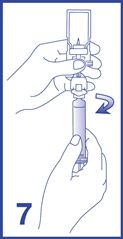
8. Label the syringe using the peel-off sticker provided.
9. For intravenous administration, immediately connect the syringe to a dose administration line (20G) and administer as directed under the Administration Instructions below. For intravesical administration, immediately connect the syringe to a sterile urinary catheter (6 French to 8 French) and administer as directed under the Administration Instructions below.
- Following reconstitution, Lumason suspension contains 1.5 to 5.6 x108microspheres/mL with 45 mcg/mL of sulfur hexafluoride.
- Use immediately after reconstitution. If the suspension is not used immediately after reconstitution, resuspend the microspheres for a few seconds by hand agitation before the suspension is drawn into the syringe. Reconstituted suspension within a vial may be used for up to 3 hours from the time of its reconstitution. Maintain the vial containing the reconstituted suspension at room temperature 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).








- Echocardiography in adults: After reconstitution, administer 2 mL as an intravenous injection (,
2.2 Recommended DosageEchocardiographyAdultsThe recommended dose of Lumason after reconstitution is 2 mL administered as an intravenous bolus injection during echocardiography. During a single examination, a second injection of 2 mL may be administered to prolong contrast enhancement. Follow each Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during echocardiography. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2 mL per injection. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the LiverAdultsThe recommended dose of Lumason after reconstitution in adult patients is 2.4 mL administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 2.4 mL may be administered, if needed. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2.4 mL per injection. Follow Lumason injection with an intravenous flush of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the Urinary TractPediatric PatientsThe recommended dose of Lumason after reconstitution is 1 mL. The bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
)2.4 Administration InstructionsInspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted suspension is milky-white, and does not contain visible particulate matter. Do not use the single-patient-use vial for more than one patient.
Intravenous AdministrationAdminister Lumason as an intravenous bolus injection.
Intravesical Administration in Pediatric Patients- Insert a sterile 6 French to 8 French urinary catheter into the bladder under sterile conditions;
- Empty the bladder of urine, and then fill the bladder with sterile 0.9% Sodium Chloride Injection, USP to approximately one third or half of its predicted total volume. The total bladder volume in children is calculated as [(age in years + 2) x 30] mL;
- Administer Lumason as an intravesical bolus injection through the urinary catheter;
- Continue filling the bladder wIith 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion.
- Immediately following the first voiding, the bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
- Echocardiography in pediatric patients: After reconstitution, administer 0.03 mL per kg as an intravenous injection up to a maximum of 2 mL per injection (,
2.2 Recommended DosageEchocardiographyAdultsThe recommended dose of Lumason after reconstitution is 2 mL administered as an intravenous bolus injection during echocardiography. During a single examination, a second injection of 2 mL may be administered to prolong contrast enhancement. Follow each Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during echocardiography. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2 mL per injection. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the LiverAdultsThe recommended dose of Lumason after reconstitution in adult patients is 2.4 mL administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 2.4 mL may be administered, if needed. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2.4 mL per injection. Follow Lumason injection with an intravenous flush of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the Urinary TractPediatric PatientsThe recommended dose of Lumason after reconstitution is 1 mL. The bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
)2.4 Administration InstructionsInspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted suspension is milky-white, and does not contain visible particulate matter. Do not use the single-patient-use vial for more than one patient.
Intravenous AdministrationAdminister Lumason as an intravenous bolus injection.
Intravesical Administration in Pediatric Patients- Insert a sterile 6 French to 8 French urinary catheter into the bladder under sterile conditions;
- Empty the bladder of urine, and then fill the bladder with sterile 0.9% Sodium Chloride Injection, USP to approximately one third or half of its predicted total volume. The total bladder volume in children is calculated as [(age in years + 2) x 30] mL;
- Administer Lumason as an intravesical bolus injection through the urinary catheter;
- Continue filling the bladder wIith 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion.
- Immediately following the first voiding, the bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
- Ultrasonography of the liver in adults: After reconstitution, administer 2.4 mL as an intravenous injection (,
2.2 Recommended DosageEchocardiographyAdultsThe recommended dose of Lumason after reconstitution is 2 mL administered as an intravenous bolus injection during echocardiography. During a single examination, a second injection of 2 mL may be administered to prolong contrast enhancement. Follow each Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during echocardiography. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2 mL per injection. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the LiverAdultsThe recommended dose of Lumason after reconstitution in adult patients is 2.4 mL administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 2.4 mL may be administered, if needed. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2.4 mL per injection. Follow Lumason injection with an intravenous flush of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the Urinary TractPediatric PatientsThe recommended dose of Lumason after reconstitution is 1 mL. The bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
)2.4 Administration InstructionsInspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted suspension is milky-white, and does not contain visible particulate matter. Do not use the single-patient-use vial for more than one patient.
Intravenous AdministrationAdminister Lumason as an intravenous bolus injection.
Intravesical Administration in Pediatric Patients- Insert a sterile 6 French to 8 French urinary catheter into the bladder under sterile conditions;
- Empty the bladder of urine, and then fill the bladder with sterile 0.9% Sodium Chloride Injection, USP to approximately one third or half of its predicted total volume. The total bladder volume in children is calculated as [(age in years + 2) x 30] mL;
- Administer Lumason as an intravesical bolus injection through the urinary catheter;
- Continue filling the bladder wIith 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion.
- Immediately following the first voiding, the bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
- Ultrasonography of the liver in pediatric patients: After reconstitution, administer 0.03 mL per kg as an intravenous injection, up to a maximum of 2.4 mL per injection (,
2.2 Recommended DosageEchocardiographyAdultsThe recommended dose of Lumason after reconstitution is 2 mL administered as an intravenous bolus injection during echocardiography. During a single examination, a second injection of 2 mL may be administered to prolong contrast enhancement. Follow each Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during echocardiography. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2 mL per injection. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the LiverAdultsThe recommended dose of Lumason after reconstitution in adult patients is 2.4 mL administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 2.4 mL may be administered, if needed. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2.4 mL per injection. Follow Lumason injection with an intravenous flush of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the Urinary TractPediatric PatientsThe recommended dose of Lumason after reconstitution is 1 mL. The bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
)2.4 Administration InstructionsInspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted suspension is milky-white, and does not contain visible particulate matter. Do not use the single-patient-use vial for more than one patient.
Intravenous AdministrationAdminister Lumason as an intravenous bolus injection.
Intravesical Administration in Pediatric Patients- Insert a sterile 6 French to 8 French urinary catheter into the bladder under sterile conditions;
- Empty the bladder of urine, and then fill the bladder with sterile 0.9% Sodium Chloride Injection, USP to approximately one third or half of its predicted total volume. The total bladder volume in children is calculated as [(age in years + 2) x 30] mL;
- Administer Lumason as an intravesical bolus injection through the urinary catheter;
- Continue filling the bladder wIith 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion.
- Immediately following the first voiding, the bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
- May repeat dose one time during a single examination (,
2.2 Recommended DosageEchocardiographyAdultsThe recommended dose of Lumason after reconstitution is 2 mL administered as an intravenous bolus injection during echocardiography. During a single examination, a second injection of 2 mL may be administered to prolong contrast enhancement. Follow each Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during echocardiography. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2 mL per injection. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the LiverAdultsThe recommended dose of Lumason after reconstitution in adult patients is 2.4 mL administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 2.4 mL may be administered, if needed. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2.4 mL per injection. Follow Lumason injection with an intravenous flush of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the Urinary TractPediatric PatientsThe recommended dose of Lumason after reconstitution is 1 mL. The bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
)2.4 Administration InstructionsInspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted suspension is milky-white, and does not contain visible particulate matter. Do not use the single-patient-use vial for more than one patient.
Intravenous AdministrationAdminister Lumason as an intravenous bolus injection.
Intravesical Administration in Pediatric Patients- Insert a sterile 6 French to 8 French urinary catheter into the bladder under sterile conditions;
- Empty the bladder of urine, and then fill the bladder with sterile 0.9% Sodium Chloride Injection, USP to approximately one third or half of its predicted total volume. The total bladder volume in children is calculated as [(age in years + 2) x 30] mL;
- Administer Lumason as an intravesical bolus injection through the urinary catheter;
- Continue filling the bladder wIith 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion.
- Immediately following the first voiding, the bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
- Follow each injection with an intravenous flush of 0.9% Sodium Chloride Injection, USP (,
2.2 Recommended DosageEchocardiographyAdultsThe recommended dose of Lumason after reconstitution is 2 mL administered as an intravenous bolus injection during echocardiography. During a single examination, a second injection of 2 mL may be administered to prolong contrast enhancement. Follow each Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during echocardiography. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2 mL per injection. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the LiverAdultsThe recommended dose of Lumason after reconstitution in adult patients is 2.4 mL administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 2.4 mL may be administered, if needed. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2.4 mL per injection. Follow Lumason injection with an intravenous flush of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the Urinary TractPediatric PatientsThe recommended dose of Lumason after reconstitution is 1 mL. The bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
)2.4 Administration InstructionsInspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted suspension is milky-white, and does not contain visible particulate matter. Do not use the single-patient-use vial for more than one patient.
Intravenous AdministrationAdminister Lumason as an intravenous bolus injection.
Intravesical Administration in Pediatric Patients- Insert a sterile 6 French to 8 French urinary catheter into the bladder under sterile conditions;
- Empty the bladder of urine, and then fill the bladder with sterile 0.9% Sodium Chloride Injection, USP to approximately one third or half of its predicted total volume. The total bladder volume in children is calculated as [(age in years + 2) x 30] mL;
- Administer Lumason as an intravesical bolus injection through the urinary catheter;
- Continue filling the bladder wIith 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion.
- Immediately following the first voiding, the bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
- Ultrasonography of the urinary tract: After reconstitution, administer 1 mL via sterile 6 to 8F urinary catheter. Bladder should be first emptied and then partially filled with 0.9% Sodium Chloride Injection, USP before injection of Lumason (,
2.2 Recommended DosageEchocardiographyAdultsThe recommended dose of Lumason after reconstitution is 2 mL administered as an intravenous bolus injection during echocardiography. During a single examination, a second injection of 2 mL may be administered to prolong contrast enhancement. Follow each Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during echocardiography. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2 mL per injection. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the LiverAdultsThe recommended dose of Lumason after reconstitution in adult patients is 2.4 mL administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 2.4 mL may be administered, if needed. Follow Lumason injection with an intravenous flush using 5 mL of 0.9% Sodium Chloride Injection, USP.
Pediatric PatientsThe recommended dose of Lumason after reconstitution in pediatric patients is 0.03 mL per kg administered as an intravenous injection during ultrasonography of the liver. During a single examination, a second injection of 0.03 mL per kg may be administered, if needed. Do not exceed 2.4 mL per injection. Follow Lumason injection with an intravenous flush of 0.9% Sodium Chloride Injection, USP.
Ultrasonography of the Urinary TractPediatric PatientsThe recommended dose of Lumason after reconstitution is 1 mL. The bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
)2.4 Administration InstructionsInspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted suspension is milky-white, and does not contain visible particulate matter. Do not use the single-patient-use vial for more than one patient.
Intravenous AdministrationAdminister Lumason as an intravenous bolus injection.
Intravesical Administration in Pediatric Patients- Insert a sterile 6 French to 8 French urinary catheter into the bladder under sterile conditions;
- Empty the bladder of urine, and then fill the bladder with sterile 0.9% Sodium Chloride Injection, USP to approximately one third or half of its predicted total volume. The total bladder volume in children is calculated as [(age in years + 2) x 30] mL;
- Administer Lumason as an intravesical bolus injection through the urinary catheter;
- Continue filling the bladder wIith 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion.
- Immediately following the first voiding, the bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
- After Lumason administration, continue filling the bladder with 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion ()
2.4 Administration InstructionsInspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted suspension is milky-white, and does not contain visible particulate matter. Do not use the single-patient-use vial for more than one patient.
Intravenous AdministrationAdminister Lumason as an intravenous bolus injection.
Intravesical Administration in Pediatric Patients- Insert a sterile 6 French to 8 French urinary catheter into the bladder under sterile conditions;
- Empty the bladder of urine, and then fill the bladder with sterile 0.9% Sodium Chloride Injection, USP to approximately one third or half of its predicted total volume. The total bladder volume in children is calculated as [(age in years + 2) x 30] mL;
- Administer Lumason as an intravesical bolus injection through the urinary catheter;
- Continue filling the bladder wIith 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion.
- Immediately following the first voiding, the bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
For injectable suspension: Lumason
is supplied in two presentations (single patient use kit or 20-vial
pack):
3-part single
patient use kit comprised of:
- one Lumason clear vial containing 25 mg of lipid-type A
sterile white lyophilized powder with headspace filled with 60.7 mg
of sulfur hexafluoride gas - one prefilled syringe containing 5 mL of 0.9% Sodium Chloride
Injection, USP (Diluent) - one Mini-Spike
20-vial pack comprised
of:
- twenty Lumason clear vials, each containing 25 mg of lipid-type
A sterile white lyophilized powder with headspace filled with 60.7
mg of sulfur hexafluoride gas - twenty Mini-Spikes
- twenty peel-off syringe labels
Following reconstitution,
Lumason is a homogeneous, milky white suspension containing 1.5 to
5.6 x 108 microspheres/mL with 45 mcg/mL
of sulfur hexafluoride.
There are no data with Lumason use in pregnant
women to inform any drug-associated risks. No adverse developmental
outcomes were observed in animal reproduction studies with administration
of sulfur hexafluoride lipid-type A microspheres in pregnant rats
and rabbits during organogenesis at doses up to at least 10 and 20
times, respectively, the maximum human dose of 4.8 mL based on body
surface area (
In the U.S. general population,
the estimated background risk of major birth defects and miscarriage
in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Lumason was administered intravenously
to rats at doses of 0.2, 1, and 5 mL/kg (approximately 0.4, 2, and
10 times the recommended maximum human dose of 4.8 mL, respectively,
based on body surface area); Lumason doses were administered daily
for about 30 consecutive days, from two weeks before pairing until
the end of organogenesis. Lumason was administered intravenously to
rabbits at doses of 0.2, 1, and 5 mL/kg (approximately 0.8, 4, and
20 times the recommended maximum human dose, respectively, based on
body surface area); Lumason doses were administered daily from gestation
day 6 to day 19 inclusive. No significant findings on the fetus were
observed.