Lurasidone Hydrochloride Prescribing Information
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Lurasidone hydrochloride tablets are not approved for the treatment of patients with dementia-related psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6- to 1.7-times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Lurasidone hydrochloride tablets are not approved for the treatment of patients with dementia-related psychosis
Antidepressants increased the risk of suicidal thoughts and behavior in pediatric and young adults in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients, and over 4,400 pediatric patients, the incidence of suicidal thoughts and behaviors in pediatric and young adult patients was greater in antidepressant-treated patients than in placebo-treated patients. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1,000 patients treated are provided in Table 2.
No suicides occurred in any of the pediatric studies. There were suicides in the adult studies, but the number was not sufficient to reach any conclusion about antidepressant drug effect on suicide.
Age Range | Drug-Placebo Difference in Number of Patients of Suicidal Thoughts or Behaviors per 1000 Patients Treated |
| Increases Compared to Placebo | |
| <18 | 14 additional patients |
| 18-24 | 5 additional patients |
| Decreases Compared to Placebo | |
| 25-64 | 1 fewer patient |
| ≥65 | 6 fewer patients |
It is unknown whether the risk of suicidal thoughts and behaviors in pediatric and young adult patients extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance studies in adults with MDD that antidepressants delay the recurrence of depression.
Monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing lurasidone hydrochloride tablets, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
Warnings and Precautions (
As with other drugs that antagonize dopamine D2receptors, lurasidone hydrochloride elevates prolactin levels.
Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotrophin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported with prolactin-elevating compounds. Long-standing hyperprolactinemia, when associated with hypogonadism, may lead to decreased bone density in both female and male patients
Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent
In short-term, placebo-controlled schizophrenia studies, the median change from baseline to endpoint in prolactin levels for lurasidone hydrochloride-treated patients was +0.4 ng/mL and was -1.9 ng/mL in the placebo-treated patients. The median change from baseline to endpoint for males was +0.5 ng/mL and for females was -0.2 ng/mL. Median changes for prolactin by dose are shown in Table 14.
Placebo | Lurasidone Hydrochloride | |||||
20 mg/day | 40 mg/day | 80 mg/day | 120 mg/day | 160 mg/day | ||
| All Patients | -1.9 (n=672) | -1.1 (n=70) | -1.4 (n=476) | -0.2 (n=495) | +3.3 (n=284) | +3.3 (n=115) |
| Females | -5.1 (n=200) | -0.7 (n=19) | -4.0 (n=149) | -0.2 (n=150) | +6.7 (n=70) | +7.1 (n=36) |
| Males | -1.3 (n=472) | -1.2 (n=51) | -0.7 (n=327) | -0.2 (n=345) | +3.1 (n=214) | +2.4 (n=79) |
The proportion of patients with prolactin elevations ≥5× upper limit of normal (ULN) was 2.8% for lurasidone hydrochloride-treated patients and = 1.0% for placebo-treated patients. The proportion of female patients with prolactin elevations ≥5x ULN was 5.7% for lurasidone hydrochloride -treated patients and = 2.0% for placebo-treated female patients. The proportion of male patients with prolactin elevations ≥5x ULN was 1.6% and 0.6% for placebo-treated male patients.
In the uncontrolled longer-term schizophrenia studies (primarily open-label extension studies), lurasidone hydrochloride was associated with a median change in prolactin of -0.9 ng/mL at week 24 (n=357), -5.3ng/mL at week 36 (n=190) and -2.2 ng/mL at week 52 (n=307).
In the short-term, placebo-controlled adolescent schizophrenia study, the median change from baseline to endpoint in prolactin levels for lurasidone hydrochloride-treated patients was +1.1 ng/mL and was +0.1 ng/mL for placebo-treated patients. For lurasidone hydrochloride-treated patients, the median change from baseline to endpoint for males was +1.0 ng/mL and for females was +2.6 ng/mL. Median changes for prolactin by dose are shown in Table 15.
Placebo | lurasidone hydrochloride 40 mg/day | lurasidone hydrochloride 80 mg/day | |
| All Patients | +0.10 (n=103) | +0.75 (n=102) | +1.20 (n=99) |
| Females | +0.70 (n=39) | +0.60 (n=42) | +4.40 (n=33) |
| Males | 0.00 (n=64) | +0.75 (n=60) | +1.00 (n=66) |
The proportion of patients with prolactin elevations ≥5x ULN was 0.5% for lurasidone hydrochloride-treated patients and 1.0% for placebo-treated patients. The proportion of female patients with prolactin elevations ≥5x ULN was 1.3% for lurasidone hydrochloride-treated patients and 0% for placebo-treated female patients. The proportion of male patients with prolactin elevations ≥5x ULN was 0% for lurasidone hydrochloride treated patients and 1.6% for placebo-treated male patients.
The median change from baseline to endpoint in prolactin levels, in the adult short-term, flexible-dosed, placebo-controlled monotherapy bipolar depression study, was +1.7 ng/mL and +3.5 ng/mL with lurasidone hydrochloride tablets 20 to 60 mg/day and 80 to 120 mg/day, respectively compared to +0.3 ng/mL with placebo-treated patients. The median change from baseline to endpoint for males was +1.5 ng/mL and for females was +3.1 ng/mL. Median changes for prolactin by dose range are shown in Table 16.
Placebo | Lurasidone Hydrochloride Tablets | ||
20 to 60 mg/day | 80 to 120 mg/day | ||
| All Patients | +0.3 (n=147) | +1.7 (n=140) | +3.5 (n=144) |
| Females | 0.0 (n=82) | +1.8 (n=78) | +5.3 (n=88) |
| Males | +0.4 (n=65) | +1.2 (n=62) | +1.9 (n=56) |
Patients were randomized to flexibly dosed lurasidone hydrochloride tablets 20 to 60 mg/day, lurasidone hydrochloride tablets 80 to 120 mg/day, or placebo
The proportion of patients with prolactin elevations ≥5x upper limit of normal (ULN) was 0.4% for lurasidone hydrochloride-treated patients and 0.0% for placebo-treated patients. The proportion of female patients with prolactin elevations ≥5x ULN was 0.6% for lurasidone hydrochloride-treated patients and 0% for placebo-treated female patients. The proportion of male patients with prolactin elevations ≥5x ULN was 0% and 0% for placebo-treated male patients.
In the uncontrolled, open-label, longer-term bipolar depression study, patients who were treated with lurasidone hydrochloride as monotherapy in the short-term and continued in the longer-term study, had a median change in prolactin of -1.15 ng/mL at week 24 (n=130).
The median change from baseline to endpoint in prolactin levels, in the adult short-term, flexible-dosed, placebo-controlled adjunctive therapy bipolar depression studies was +2.8 ng/mL with lurasidone hydrochloride tablets 20 to 120 mg/day compared to 0.0 ng/mL with placebo-treated patients. The median change from baseline to endpoint for males was +2.4 ng/mL and for females was +3.2 ng/mL. Median changes for prolactin across the dose range are shown in Table 17.
Placebo | Lurasidone Hydrochloride Tablets 20 to 120 mg/day | |
| All Patients | 0.0 (n=301) | +2.8 (n=321) |
| Females | +0.4 (n=156) | +3.2 (n=162) |
| Males | -0.1 (n=145) | +2.4 (n=159) |
Patients were randomized to flexibly dosed lurasidone hydrochloride tablets 20 to 120 mg/day or placebo as adjunctive therapy with lithium or valproate.
The proportion of patients with prolactin elevations ≥5x upper limit of normal (ULN) was 0.0% for lurasidone hydrochloride-treated patients and 0.0% for placebo-treated patients. The proportion of female patients with prolactin elevations ≥5x ULN was 0% for lurasidone hydrochloride-treated patients and 0% for placebo-treated female patients. The proportion of male patients with prolactin elevations ≥5x ULN was 0% and 0% for placebo-treated male patients.
In the uncontrolled, open-label, longer-term bipolar depression study, patients who were treated with lurasidone hydrochloride, as adjunctive therapy with either lithium or valproate, in the short-term and continued in the longer-term study, had a median change in prolactin of -2.9 ng/mL at week 24 (n=88).
In the 6-week, placebo-controlled bipolar depression study with pediatric patients 10 to 17 years, the median change from baseline to endpoint in prolactin levels for lurasidone hydrochloride-treated patients was +1.10 ng/mL and was +0.50 ng/mL for placebo-treated patients. For lurasidone hydrochloride-treated patients, the median change from baseline to endpoint for males was +0.85 ng/mL and for females was +2.50 ng/mL. Median changes for prolactin are shown in Table 18.
Placebo | Lurasidone Hydrochloride 20 to 80 mg/day | |
| All Patients | +0.50 (n=157) | +1.10 (n=165) |
| Females | +0.55 (n=78) | +2.50 (n=83) |
| Males | +0.50 (n=79) | +0.85 (n=82) |
The proportion of patients with prolactin elevations ≥5x ULN was 0% for lurasidone hydrochloride-treated patients and 0.6% for placebo-treated patients. The proportion of female patients with prolactin elevations ≥5x ULN was 0% for lurasidone hydrochloride-treated patients and 1.3% for placebo-treated female patients. No male patients in the placebo or lurasidone hydrochloride treatment groups had prolactin elevations ≥5x ULN.
In a 104-week, open-label study of pediatric patients with schizophrenia, bipolar depression, or autistic disorder, the median changes from baseline to endpoint in serum prolactin levels were -0.20 ng/mL (all patients), -0.30 ng/mL (females), and -0.05 ng/mL (males). The proportions of patients with a markedly high prolactin level (≥5 times the upper limit of normal) at any time during open-label treatment were 2% (all patients), 3% (females), and 1% (males).
Adverse events among females in this trial that are potentially prolactin-related include galactorrhea (0.6%). Among male patients in this study, decreased libido was reported in one patient (0.2%) and there were no reports of impotence, gynecomastia, or galactorrhea.
Lurasidone hydrochloride tablets are indicated for:
• Treatment of adult and adolescent patients (13 to 17 years) with schizophrenia
The efficacy of lurasidone hydrochloride for the treatment of schizophrenia was established in five short-term (6-week), placebo-controlled studies in adult patients (mean age of 38.4 years, range 18-72) who met DSM-IV criteria for schizophrenia. An active-control arm (olanzapine or quetiapine extended-release) was included in two studies to assess assay sensitivity.
Several instruments were used for assessing psychiatric signs and symptoms in these studies:
1. Positive and Negative Syndrome Scale (PANSS), is a multi-item inventory of general psychopathology used to evaluate the effects of drug treatment in schizophrenia. PANSS total scores may range from 30 to 210.
2. Brief Psychiatric Rating Scale derived (BPRSd), derived from the PANSS, is a multi-item inventory primarily focusing on positive symptoms of schizophrenia, whereas the PANSS includes a wider range of positive, negative and other symptoms of schizophrenia. The BPRSd consists of 18 items rated on a scale of 1 (not present) to 7 (severe). BPRSd scores may range from 18 to 126.
3. The Clinical Global Impression severity scale (CGI-S) is a clinician-rated scale that measures the subject’s current illness state on a 1- to 7-point scale.
The endpoint associated with each instrument is change from baseline in the total score to the end of week 6. These changes are then compared to placebo changes for the drug and control groups.
The results of the studies follow:
1. Study 1: In a 6-week, placebo-controlled trial (N=145) involving two fixed doses of lurasidone hydrochloride (40 or 120 mg/day), both doses of lurasidone hydrochloride at Endpoint were superior to placebo on the BPRSd total score, and the CGI-S.
2. Study 2: In a 6-week, placebo-controlled trial (N=180) involving a fixed dose of lurasidone hydrochloride (80 mg/day), lurasidone hydrochloride at Endpoint was superior to placebo on the BPRSd total score, and the CGI-S.
3. Study 3: In a 6-week, placebo- and active-controlled trial (N=473) involving two fixed doses of lurasidone hydrochloride (40 or 120 mg/day) and an active control (olanzapine), both lurasidone hydrochloride doses and the active control at Endpoint were superior to placebo on the PANSS total score, and the CGI-S.
4. Study 4: In a 6-week, placebo-controlled trial (N=489) involving three fixed doses of lurasidone hydrochloride (40, 80 or 120 mg/day), only the 80 mg/day dose of lurasidone hydrochloride at Endpoint was superior to placebo on the PANSS total score, and the CGI-S.
5. Study 5: In a 6-week, placebo- and active-controlled trial (N=482) involving two fixed doses of lurasidone hydrochloride (80 or 160 mg/day) and an active control (quetiapine extended-release), both lurasidone hydrochloride doses and the active control at Endpoint were superior to placebo on the PANSS total score, and the CGI-S.
Thus, the efficacy of lurasidone hydrochloride at doses of 40, 80, 120 and 160 mg/day has been established (Table 35).
Primary Efficacy Measure: BPRSd | ||||
Study | Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Differencea(95% CI) |
| 1 | lurasidone hydrochloride (40 mg/day)* | 54.2 (8.8) | -9.4 (1.6) | -5.6 (-9.8, -1.4) |
| lurasidone hydrochloride (120 mg/day)* | 52.7 (7.6) | -11.0 (1.6) | -6.7 (-11.0, -2.5) | |
| Placebo | 54.7 (8.1) | -3.8 (1.6) | -- | |
| 2 | lurasidone hydrochloride (80 mg/day)* | 55.1 (6.0) | -8.9 (1.3) | -4.7 (-8.3, -1.1) |
| Placebo | 56.1 (6.8) | -4.2 (1.4) | -- | |
Primary Efficacy Measure: PANSS | ||||
| 3 | lurasidone hydrochloride (40 mg/day)* | 96.6 (10.7) | -25.7 (2.0) | -9.7 (-15.3, -4.1) |
| lurasidone hydrochloride (120 mg/day)* | 97.9 (11.3) | -23.6 (2.1) | -7.5 (-13.4, -1.7) | |
| Olanzapine (15 mg/day)*b | 96.3 (12.2) | -28.7 (1.9) | -12.6 (-18.2, -7.9) | |
| Placebo | 95.8 (10.8) | -16.0 (2.1) | -- | |
| 4 | lurasidone hydrochloride (40 mg/day) | 96.5 (11.5) | -19.2 (1.7) | -2.1 (-7.0, 2.8) |
| lurasidone hydrochloride (80 mg/day)* | 96.0 (10.8) | -23.4 (1.8) | -6.4 (-11.3, -1.5) | |
| lurasidone hydrochloride (120 mg/day) | 96.0 (9.7) | -20.5 (1.8) | -3.5(-8.4,1.4) | |
| Placebo | 96.8 (11.1) | -17.0 (1.8) | -- | |
| 5 | lurasidone hydrochloride (80 mg/day)* | 97.7 (9.7) | -22.2 (1.8) | -11.9 (-16.9, -6.9) |
| lurasidone hydrochloride (160 mg/day)* | 97.5 (11.8) | -26.5 (1.8) | -16.2 (-21.2, 11.2) | |
| Quetiapine Extended-release (600 mg/day)*b | 97.7 (10.2) | -27.8 (1.8) | -17.5 (-22.5, -12.4) | |
| Placebo | 96.6 (10.2) | -10.3 (1.8) | -- | |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, unadjusted for multiple comparisons.
a Difference (drug minus placebo) in least-squares mean change from baseline. b Included for assay sensitivity.
* Doses statistically significantly superior to placebo.
Examination of population subgroups based on age (there were few patients over 65), gender and race did not reveal any clear evidence of differential responsiveness.
The efficacy of lurasidone hydrochloride, was established in a 6-week, multicenter, randomized, double-blind, placebo-controlled study of adolescents (13 to 17 years) who met DSM-IV-TR criteria for schizophrenia (N=326). Patients were randomized to one of two fixed-doses of lurasidone hydrochloride (40 or 80 mg/day) or placebo.
The primary rating instrument used to assess psychiatric signs and symptoms was the PANSS. The key secondary instrument was the CGI-S.
For both dose groups, lurasidone hydrochloride was superior to placebo in reduction of PANSS and CGI-S scores at Week 6. On average, the 80 mg/day dose did not provide additional benefit compared to the 40 mg/day dose.
The primary efficacy results are provided in Table 36.
Primary Efficacy Measure: PANSS | |||
Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Differencea(95%CI) |
| lurasidone hydrochloride (40 mg/day)* | 94.5 (10.97) | -18.6 (1.59) | -8.0 (-12.4, -3.7) |
| lurasidone hydrochloride (80 mg/day)* | 94.0 (11.12) | -18.3 (1.60) | -7.7 (-12.1, -3.4) |
| Placebo | 92.8 (11.08) | -10.5 (1.59) | -- |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, unadjusted for multiple comparisons.
a Difference (drug minus placebo) in least-squares mean change from baseline.
* Doses statistically significantly superior to placebo.
• Monotherapy treatment of adult and pediatric patients (10 to 17 years) with major depressive episode associated with bipolar I disorder (bipolar depression)
The efficacy of lurasidone hydrochloride, as monotherapy, was established in a 6-week, multicenter, randomized, double-blind, placebo-controlled study of adult patients (mean age of 41.5 years, range 18 to 74) who met DSM-IV-TR criteria for major depressive episodes associated with bipolar I disorder, with or without rapid cycling, and without psychotic features (N=485). Patients were randomized to one of two flexible-dose ranges of lurasidone hydrochloride tablets (20 to 60 mg/day, or 80 to 120 mg/day) or placebo.
The primary rating instrument used to assess depressive symptoms in this study was the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item clinician-rated scale with total scores ranging from 0 (no depressive features) to 60 (maximum score). The primary endpoint was the change from baseline in MADRS score at Week 6. The key secondary instrument was the Clinical Global Impression-Bipolar-Severity of Illness scale (CGI-BP-S), a clinician-rated scale that measures the subject's current illness state on a 7-point scale, where a higher score is associated with greater illness severity.
For both dose groups, lurasidone hydrochloride was superior to placebo in reduction of MADRS and CGI-BP-S scores at Week 6. The primary efficacy results are provided in Table 37. The high dose range (80 to 120 mg per day) did not provide additional efficacy on average, compared to the low dose range (20 to 60 mg per day).
The efficacy of lurasidone hydrochloride, as an adjunctive therapy with lithium or valproate, was established in a 6-week, multicenter, randomized, double-blind, placebo-controlled study of adult patients (mean age of 41.7 years, range 18 to 72) who met DSM-IV-TR criteria for major depressive episodes associated with bipolar I disorder, with or without rapid cycling, and without psychotic features (N=340). Patients who remained symptomatic after treatment with lithium or valproate were randomized to flexibly dosed lurasidone hydrochloride tablets 20 to 120 mg/day or placebo.
The primary rating instrument used to assess depressive symptoms in this study was the MADRS. The primary endpoint was the change from baseline in MADRS score at Week 6. The key secondary instrument was the CGI-BP-S scale.
Lurasidone hydrochloride was superior to placebo in reduction of MADRS and CGI-BP-S scores at Week 6, as an adjunctive therapy with lithium or valproate (Table 37).
Primary Efficacy Measure: MADRS | ||||
Study | Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo- subtracted Differencea(95% CI) |
| Monotherapy study | lurasidone hydrochloride tablets (20-60 mg/day)* | 30.3 (5.0) | -15.4 (0.8) | -4.6 (-6.9, -2.3) |
| lurasidone hydrochloride tablets (80-120 mg/day)* | 30.6 (4.9) | -15.4 (0.8) | -4.6 (-6.9, -2.3) | |
Placebo | 30.5 (5.0) | -10.7 (0.8) | -- | |
| Adjunctive Therapy study | lurasidone hydrochloride tablets (20-120 mg/day)* + lithium or valproate | 30.6 (5.3) | -17.1 (0.9) | -3.6 (-6.0, -1.1) |
| Placebo + lithium or valproate | 30.8 (4.8) | -13.5 (0.9) | -- | |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, unadjusted for multiple comparisons.
aDifference (drug minus placebo) in least-squares mean change from baseline.
* Treatment group statistically significantly superior to placebo.
The efficacy of lurasidone hydrochloride was established in a 6-week, multicenter, randomized, double-blind, placebo-controlled study of pediatric patients (10 to 17 years) who met DSM-5 criteria for a major depressive episode associated with bipolar I disorder, with or without rapid cycling, and without psychotic features (N=343). Patients were randomized to flexibly dosed lurasidone hydrochloride 20 to 80 mg/day or placebo. At the end of the clinical study, most patients (67%) received 20 mg/day or 40 mg/day.
The primary rating scale used to assess depressive symptoms in this study was the Children’s Depression Rating Scale, Revised (CDRS-R) total score. The CDRS-R is a 17-item clinician-rated scale with total scores ranging from 17 to 113. The primary endpoint was the change from baseline in CDRS-R score at Week 6. The key secondary endpoint was the change from baseline in CGI-BP-S depression score.
Lurasidone hydrochloride was superior to placebo in reduction of CDRS-R total score and CGI-BP-S depression score at Week 6. The primary efficacy results are provided in Table 38.
Primary Efficacy Measure: CDRS-R | |||
Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo- subtracted Differencea(95% CI) |
| Lurasidone hydrochloride (20 to 80 mg/day)* | 59.2 (8.24) | -21.0 (1.06) | -5.7 (-8.4, -3.0) |
| Placebo | 58.6 (8.26) | -15.3 (1.08) | -- |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, unadjusted for multiple comparisons.
aDifference (drug minus placebo) in least-squares mean change from baseline.
*Treatment group statistically significantly superior to placebo.
• Adjunctive treatment with lithium or valproate in adult patients with major depressive episode associated with bipolar I disorder (bipolar depression)
The efficacy of lurasidone hydrochloride, as monotherapy, was established in a 6-week, multicenter, randomized, double-blind, placebo-controlled study of adult patients (mean age of 41.5 years, range 18 to 74) who met DSM-IV-TR criteria for major depressive episodes associated with bipolar I disorder, with or without rapid cycling, and without psychotic features (N=485). Patients were randomized to one of two flexible-dose ranges of lurasidone hydrochloride tablets (20 to 60 mg/day, or 80 to 120 mg/day) or placebo.
The primary rating instrument used to assess depressive symptoms in this study was the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item clinician-rated scale with total scores ranging from 0 (no depressive features) to 60 (maximum score). The primary endpoint was the change from baseline in MADRS score at Week 6. The key secondary instrument was the Clinical Global Impression-Bipolar-Severity of Illness scale (CGI-BP-S), a clinician-rated scale that measures the subject's current illness state on a 7-point scale, where a higher score is associated with greater illness severity.
For both dose groups, lurasidone hydrochloride was superior to placebo in reduction of MADRS and CGI-BP-S scores at Week 6. The primary efficacy results are provided in Table 37. The high dose range (80 to 120 mg per day) did not provide additional efficacy on average, compared to the low dose range (20 to 60 mg per day).
The efficacy of lurasidone hydrochloride, as an adjunctive therapy with lithium or valproate, was established in a 6-week, multicenter, randomized, double-blind, placebo-controlled study of adult patients (mean age of 41.7 years, range 18 to 72) who met DSM-IV-TR criteria for major depressive episodes associated with bipolar I disorder, with or without rapid cycling, and without psychotic features (N=340). Patients who remained symptomatic after treatment with lithium or valproate were randomized to flexibly dosed lurasidone hydrochloride tablets 20 to 120 mg/day or placebo.
The primary rating instrument used to assess depressive symptoms in this study was the MADRS. The primary endpoint was the change from baseline in MADRS score at Week 6. The key secondary instrument was the CGI-BP-S scale.
Lurasidone hydrochloride was superior to placebo in reduction of MADRS and CGI-BP-S scores at Week 6, as an adjunctive therapy with lithium or valproate (Table 37).
Primary Efficacy Measure: MADRS | ||||
Study | Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo- subtracted Differencea(95% CI) |
| Monotherapy study | lurasidone hydrochloride tablets (20-60 mg/day)* | 30.3 (5.0) | -15.4 (0.8) | -4.6 (-6.9, -2.3) |
| lurasidone hydrochloride tablets (80-120 mg/day)* | 30.6 (4.9) | -15.4 (0.8) | -4.6 (-6.9, -2.3) | |
Placebo | 30.5 (5.0) | -10.7 (0.8) | -- | |
| Adjunctive Therapy study | lurasidone hydrochloride tablets (20-120 mg/day)* + lithium or valproate | 30.6 (5.3) | -17.1 (0.9) | -3.6 (-6.0, -1.1) |
| Placebo + lithium or valproate | 30.8 (4.8) | -13.5 (0.9) | -- | |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, unadjusted for multiple comparisons.
aDifference (drug minus placebo) in least-squares mean change from baseline.
* Treatment group statistically significantly superior to placebo.
The efficacy of lurasidone hydrochloride was established in a 6-week, multicenter, randomized, double-blind, placebo-controlled study of pediatric patients (10 to 17 years) who met DSM-5 criteria for a major depressive episode associated with bipolar I disorder, with or without rapid cycling, and without psychotic features (N=343). Patients were randomized to flexibly dosed lurasidone hydrochloride 20 to 80 mg/day or placebo. At the end of the clinical study, most patients (67%) received 20 mg/day or 40 mg/day.
The primary rating scale used to assess depressive symptoms in this study was the Children’s Depression Rating Scale, Revised (CDRS-R) total score. The CDRS-R is a 17-item clinician-rated scale with total scores ranging from 17 to 113. The primary endpoint was the change from baseline in CDRS-R score at Week 6. The key secondary endpoint was the change from baseline in CGI-BP-S depression score.
Lurasidone hydrochloride was superior to placebo in reduction of CDRS-R total score and CGI-BP-S depression score at Week 6. The primary efficacy results are provided in Table 38.
Primary Efficacy Measure: CDRS-R | |||
Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo- subtracted Differencea(95% CI) |
| Lurasidone hydrochloride (20 to 80 mg/day)* | 59.2 (8.24) | -21.0 (1.06) | -5.7 (-8.4, -3.0) |
| Placebo | 58.6 (8.26) | -15.3 (1.08) | -- |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, unadjusted for multiple comparisons.
aDifference (drug minus placebo) in least-squares mean change from baseline.
*Treatment group statistically significantly superior to placebo.
Lurasidone hydrochloride tablets should be taken with food (at least 350 calories). Administration with food substantially increases the absorption of lurasidone hydrochloride tablets (
Lurasidone hydrochloride tablets should be taken with food (at least 350 calories). Administration with food substantially increases the absorption of lurasidone hydrochloride tablets. Administration with food increases the AUC approximately 2-fold and increases the Cmaxapproximately 3-fold. In the clinical studies, lurasidone hydrochloride tablets were administered with food
The effectiveness of lurasidone hydrochloride tablets for longer-term use, that is, for more than 6 weeks, has not been established in controlled studies. Therefore, the physician who elects to use lurasidone hydrochloride tablets for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient
The activity of lurasidone hydrochloride is primarily due to the parent drug. The pharmacokinetics of lurasidone hydrochloride is dose-proportional within a total daily dose range of 20 mg to 160 mg. Steady-state concentrations of lurasidone hydrochloride tablets are reached within 7 days of starting lurasidone hydrochloride.
Following administration of 40 mg of lurasidone hydrochloride, the mean (%CV) elimination half-life was 18 (7) hours.
In a food effect study, lurasidone hydrochloride mean Cmaxand AUC were about 3-times and 2-times, respectively, when administered with food compared to the levels observed under fasting conditions. Lurasidone hydrochloride exposure was not affected as meal size was increased from 350 to 1,000 calories and was independent of meal fat content
In clinical studies, establishing the safety and efficacy of lurasidone hydrochloride, patients were instructed to take their daily dose with food
Total excretion of radioactivity in urine and feces combined was approximately 89%, with about 80% recovered in feces and 9% recovered in urine, after a single dose of [14C]-labeled lurasidone hydrochloride.
Following administration of 40 mg of lurasidone hydrochloride, the mean (%CV) apparent clearance was 3,902 (18.0) mL/min.
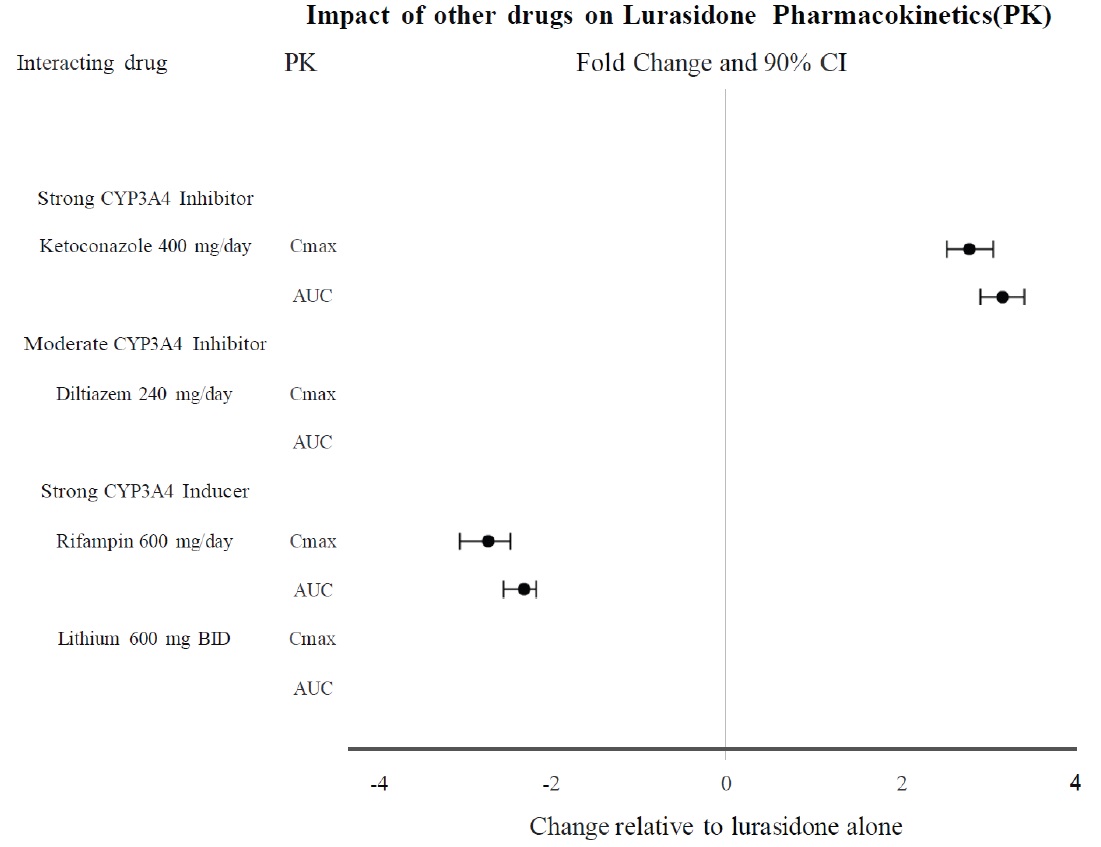
Effects of other drugs on the exposure of lurasidone are summarized in Figure 1. A population PK analyses concluded that coadministration of lithium 300-2400 mg/day or valproate 300-2000 mg/day with lurasidone for up to 6 weeks has minimal effect on lurasidone exposure.
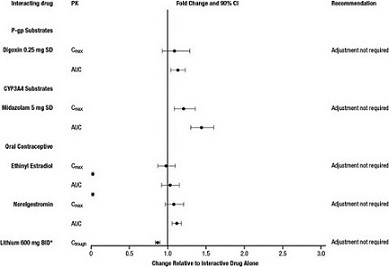
And the effects of lurasidone hydrochloride on the exposures of other drugs are summarized in Figure 2. A population PK analyses concluded that coadministration of lurasidone has minimal effect on lithium and valproate exposure when it is coadministered with lithium 300-2400 mg/day or valproate 300-2000 mg/day.
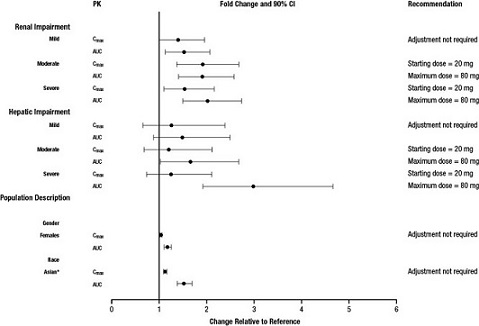
The effect of intrinsic patient factors on the pharmacokinetics of lurasidone hydrochloride is presented in Figure 3.
Lurasidone hydrochloride exposure (i.e., steady-state Cmax and AUC) in children and adolescent patients (10 to 17 years of age) was generally similar to that in adults across the dose range from 40 to 160 mg, without adjusting for body weight.



| Indication | Starting Dose | Recommended Dose |
| Schizophrenia – adults (2.1) | 40 mg per day | 40 mg to 160 mg per day |
| Schizophrenia – adolescents (13 to 17 years) (2.1) | 40 mg per day | 40 mg to 80 mg per day |
| Bipolar Depression - adults (2.2) | 20 mg per day | 20 mg to 120 mg per day |
| Bipolar Depression – pediatric patients (10 to 17 years) (2.2) | 20 mg per day | 20 mg to 80 mg per day |
•
Dose adjustment is recommended in moderate (creatinine clearance: 30 to <50 mL/min) and severe renal impairment (creatinine clearance <30 mL/min) patients. The recommended starting dose is 20 mg per day. The dose in these patients should not exceed 80 mg per day
Reduce the maximum recommended dosage in patients with moderate or severe renal impairment (CLcr<50 mL/minute). Patients with impaired renal function (CLcr<50 mL/minute) had higher exposure to lurasidone than patients with normal renal function
•
Dose adjustment is recommended in moderate (Child-Pugh Score = 7 to 9) and severe hepatic impairment (Child-Pugh Score = 10 to 15) patients. The recommended starting dose is 20 mg per day. The dose in moderate hepatic impairment patients should not exceed 80 mg per day and the dose in severe hepatic impairment patients should not exceed 40 per mg/day
Reduce the maximum recommended dosage in patients with moderate to severe hepatic impairment (Child-Pugh score ≥7). Patients with moderate to severe hepatic impairment (Child- Pugh score ≥7) generally had higher exposure to lurasidone than patients with normal hepatic function
•
Lurasidone hydrochloride tablets should not be used concomitantly with a strong CYP3A4 inhibitor (e.g., ketoconazole, clarithromycin, ritonavir, voriconazole, mibefradil, etc.)
If lurasidone hydrochloride tablets are being prescribed and a moderate CYP3A4 inhibitor (e.g. diltiazem, atazanavir, erythromycin, fluconazole, verapamil, etc.) is added to the therapy, the lurasidone hydrochloride tablets dose should be reduced to half of the original dose level. Similarly, if a moderate CYP3A4 inhibitor is being prescribed and lurasidone hydrochloride tablets are added to the therapy, the recommended starting dose of lurasidone hydrochloride tablets is 20 mg per day, and the maximum recommended dose of lurasidone hydrochloride tablets is 80 mg per day
Grapefruit and grapefruit juice should be avoided in patients taking lurasidone hydrochloride tablets, since these may inhibit CYP3A4 and alter lurasidone hydrochloride tablets concentrations
Lurasidone hydrochloride tablets should not be used concomitantly with a strong CYP3A4 inducer (e.g., rifampin, avasimibe, St. John's wort, phenytoin, carbamazepine, etc.)
Strong CYP3A4 Inhibitors | |
| Clinical Impact: | Concomitant use of lurasidone hydrochloride tablets with strong CYP3A4 inhibitors increased the exposure of lurasidone compared to the use of lurasidone hydrochloride tablets alone [ see Clinical Pharmacology (12.3)]. |
| Intervention: | Lurasidone hydrochloride tablets should not be used concomitantly with strong CYP3A4 inhibitors [ see Contraindications (4)]. |
| Examples: | Ketoconazole, clarithromycin, ritonavir, voriconazole, mibefradil |
Moderate CYP3A4 Inhibitors | |
| Clinical Impact: | Concomitant use of lurasidone hydrochloride tablets with moderate CYP3A4 inhibitors increased the exposure of lurasidone compared to the use of lurasidone hydrochloride tablets alone [ see Clinical Pharmacology (12.3)]. |
| Intervention: | Lurasidone hydrochloride dose should be reduced to half of the original level when used concomitantly with moderate inhibitors of CYP3A4 [ see Dosage and Administration (2.6)]. |
| Examples: | Diltiazem, atazanavir, erythromycin, fluconazole, verapamil |
Strong CYP3A4 Inducers | |
| Clinical Impact: | Concomitant use of lurasidone hydrochloride tablets with strong CYP3A4 inducers decreased the exposure of lurasidone compared to the use of lurasidone hydrochloride tablets alone [ see Clinical Pharmacology (12.3)]. |
| Intervention: | Lurasidone hydrochloride tablets should not be used concomitantly with strong CYP3A4 inducers [ see Contraindications (4)]. |
| Examples: | Rifampin, avasimibe, St. John’s wort, phenytoin, carbamazepine |
Moderate CYP3A4 Inducers | |
| Clinical Impact: | Concomitant use of lurasidone hydrochloride tablets with moderate CYP3A4 inducers decreased the exposure of lurasidone compared to the use of lurasidone hydrochloride tablets alone [ see Clinical Pharmacology (12.3)]. |
| Intervention: | Lurasidone hydrochloride tablets dose should be increased when used concomitantly with moderate inducers of CYP3A4 [ see Dosage and Administration (2.6)]. |
| Examples: | Bosentan, efavirenz, etravirine, modafinil, nafcillin |
•
Lurasidone hydrochloride tablets should not be used concomitantly with a strong CYP3A4 inhibitor (e.g., ketoconazole, clarithromycin, ritonavir, voriconazole, mibefradil, etc.)
If lurasidone hydrochloride tablets are being prescribed and a moderate CYP3A4 inhibitor (e.g. diltiazem, atazanavir, erythromycin, fluconazole, verapamil, etc.) is added to the therapy, the lurasidone hydrochloride tablets dose should be reduced to half of the original dose level. Similarly, if a moderate CYP3A4 inhibitor is being prescribed and lurasidone hydrochloride tablets are added to the therapy, the recommended starting dose of lurasidone hydrochloride tablets is 20 mg per day, and the maximum recommended dose of lurasidone hydrochloride tablets is 80 mg per day
Grapefruit and grapefruit juice should be avoided in patients taking lurasidone hydrochloride tablets, since these may inhibit CYP3A4 and alter lurasidone hydrochloride tablets concentrations
Lurasidone hydrochloride tablets should not be used concomitantly with a strong CYP3A4 inducer (e.g., rifampin, avasimibe, St. John's wort, phenytoin, carbamazepine, etc.)
Strong CYP3A4 Inhibitors | |
| Clinical Impact: | Concomitant use of lurasidone hydrochloride tablets with strong CYP3A4 inhibitors increased the exposure of lurasidone compared to the use of lurasidone hydrochloride tablets alone [ see Clinical Pharmacology (12.3)]. |
| Intervention: | Lurasidone hydrochloride tablets should not be used concomitantly with strong CYP3A4 inhibitors [ see Contraindications (4)]. |
| Examples: | Ketoconazole, clarithromycin, ritonavir, voriconazole, mibefradil |
Moderate CYP3A4 Inhibitors | |
| Clinical Impact: | Concomitant use of lurasidone hydrochloride tablets with moderate CYP3A4 inhibitors increased the exposure of lurasidone compared to the use of lurasidone hydrochloride tablets alone [ see Clinical Pharmacology (12.3)]. |
| Intervention: | Lurasidone hydrochloride dose should be reduced to half of the original level when used concomitantly with moderate inhibitors of CYP3A4 [ see Dosage and Administration (2.6)]. |
| Examples: | Diltiazem, atazanavir, erythromycin, fluconazole, verapamil |
Strong CYP3A4 Inducers | |
| Clinical Impact: | Concomitant use of lurasidone hydrochloride tablets with strong CYP3A4 inducers decreased the exposure of lurasidone compared to the use of lurasidone hydrochloride tablets alone [ see Clinical Pharmacology (12.3)]. |
| Intervention: | Lurasidone hydrochloride tablets should not be used concomitantly with strong CYP3A4 inducers [ see Contraindications (4)]. |
| Examples: | Rifampin, avasimibe, St. John’s wort, phenytoin, carbamazepine |
Moderate CYP3A4 Inducers | |
| Clinical Impact: | Concomitant use of lurasidone hydrochloride tablets with moderate CYP3A4 inducers decreased the exposure of lurasidone compared to the use of lurasidone hydrochloride tablets alone [ see Clinical Pharmacology (12.3)]. |
| Intervention: | Lurasidone hydrochloride tablets dose should be increased when used concomitantly with moderate inducers of CYP3A4 [ see Dosage and Administration (2.6)]. |
| Examples: | Bosentan, efavirenz, etravirine, modafinil, nafcillin |
Lurasidone hydrochloride tablets are available as follows:
20 mg tablets are white colored, round shaped, biconvex, film-coated tablets, debossed with “20” on one side and “ML” on other side.
40 mg tablets are white colored, round shaped, biconvex, film-coated tablets, debossed with “40” on one side and “ML” on other side.
60 mg tablets are white colored, oval shaped, biconvex, film-coated tablets, debossed with “60” on one side and “ML” on other side.
80 mg tablets are white colored, oval shaped, biconvex, film-coated tablets, debossed with “80” on one side and “ML” on other side.
120 mg tablets are white colored, oval shaped, biconvex, film-coated tablets, debossed with “120” on one side and “ML” on other side.
•
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to lurasidone hydrochloride during pregnancy. For more information, contact the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs during the third trimester of pregnancy. These symptoms have varied in severity. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately.
Pregnant rats were treated with oral lurasidone at doses of 3, 10, and 25 mg/kg/day during the period of organogenesis. These doses are 0.2, 0.6, and 1.5 times the MRHD of 160 mg/day based on mg/m2body surface area. No teratogenic or embryo-fetal effects were observed up to 1.5 times the MRHD of 160 mg/day, based on mg/m2.
Pregnant rabbits were treated with oral lurasidone at doses of 2, 10, and 50 mg/kg/day during the period of organogenesis. These doses are 0.2, 1.2 and 6 times the MRHD of 160 mg/day based on mg/m2. No teratogenic or embryo-fetal effects were observed up to 6 times the MRHD of 160 mg/day based on mg/m2.
Pregnant rats were treated with oral lurasidone at doses of 0.4, 2, and 10 mg/kg/day during the periods of organogenesis and lactation. These doses are 0.02, 0.1 and 0.6 times the MRHD of 160 mg/day based on mg/m2. No pre- and postnatal developmental effects were observed up to 0.6 times the MRHD of 160 mg/day, based on mg/m2.