Lyrica
(Pregabalin)Lyrica Prescribing Information
Warnings and Precautions (
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED‑treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed.
Table 3 shows absolute and relative risk by indication for all evaluated AEDs.
Indication | Placebo Patients with Events Per 1000 Patients | Drug Patients with Events Per 1000 Patients | Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients | Risk Difference: Additional Drug Patients with Events Per 1000 Patients |
Epilepsy | 1.0 | 3.4 | 3.5 | 2.4 |
Psychiatric | 5.7 | 8.5 | 1.5 | 2.9 |
Other | 1.0 | 1.8 | 1.9 | 0.9 |
Total | 2.4 | 4.3 | 1.8 | 1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing LYRICA or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
As with all antiepileptic drugs (AEDs), withdraw LYRICA gradually to minimize the potential of increased seizure frequency in patients with seizure disorders.
If LYRICA is discontinued, taper the drug gradually over a minimum of 1 week rather than discontinue the drug abruptly.
LYRICA is indicated for:
• Management of neuropathic pain associated with diabetic peripheral neuropathy• Management of postherpetic neuralgia• Adjunctive therapy for the treatment of partial‑onset seizures in patients 1 month of age and older• Management of fibromyalgia• Management of neuropathic pain associated with spinal cord injury
• For adult indications, begin dosing at 150 mg/day. For partial‑onset seizure dosing in pediatric patients 1 month of age and older, refer to section 2.4. (,2.2 Neuropathic Pain Associated with Diabetic Peripheral Neuropathy in AdultsThe maximum recommended dose of LYRICA is 100 mg three times a day (300 mg/day) in patients with creatinine clearance of at least 60 mL/min. Begin dosing at 50 mg three times a day (150 mg/day). The dose may be increased to 300 mg/day within 1 week based on efficacy and tolerability.
Although LYRICA was also studied at 600 mg/day, there is no evidence that this dose confers additional significant benefit and this dose was less well tolerated. In view of the dose-dependent adverse reactions, treatment with doses above 300 mg/day is not recommended
[see Adverse Reactions (6.1)].,2.3 Postherpetic Neuralgia in AdultsThe recommended dose of LYRICA is 75 to 150 mg two times a day, or 50 to 100 mg three times a day (150 to 300 mg/day) in patients with creatinine clearance of at least 60 mL/min. Begin dosing at 75 mg two times a day, or 50 mg three times a day (150 mg/day). The dose may be increased to 300 mg/day within 1 week based on efficacy and tolerability.
Patients who do not experience sufficient pain relief following 2 to 4 weeks of treatment with 300 mg/day, and who are able to tolerate LYRICA, may be treated with up to 300 mg two times a day, or 200 mg three times a day (600 mg/day). In view of the dose-dependent adverse reactions and the higher rate of treatment discontinuation due to adverse reactions, reserve dosing above 300 mg/day for those patients who have on-going pain and are tolerating 300 mg daily
[see Adverse Reactions (6.1)].,2.4 Adjunctive Therapy for Partial-Onset Seizures in Patients 1 Month of Age and OlderThe recommended dosages for adults and pediatric patients 1 month of age and older are included in Table 1. Administer the total daily dosage orally in two or three divided doses as indicated in Table 1. In pediatric patients, the recommended dosing regimen is dependent upon body weight. Based on clinical response and tolerability, dosage may be increased, approximately weekly.
Table 1. Recommended Dosage for Adults and Pediatric Patients 1 Month and Older Age and Body WeightRecommended Initial DosageRecommended Maximum DosageFrequency of AdministrationAdults (17 years and older)
150 mg/day
600 mg/day
2 or 3 divided doses
Pediatric patients weighing 30 kg or more
2.5 mg/kg/day
10 mg/kg/day (not to exceed 600 mg/day)
2 or 3 divided doses
Pediatric patients weighing less than 30 kg
3.5 mg/kg/day
14 mg/kg/day
1 month to less than 4 years of age:
3 divided doses4 years of age and older:
2 or 3 divided dosesBoth the efficacy and adverse event profiles of LYRICA have been shown to be dose-related.
The effect of dose escalation rate on the tolerability of LYRICA has not been formally studied.
The efficacy of adjunctive LYRICA in patients taking gabapentin has not been evaluated in controlled trials. Consequently, dosing recommendations for the use of LYRICA with gabapentin cannot be offered.
,2.5 Management of Fibromyalgia in AdultsThe recommended dose of LYRICA for fibromyalgia is 300 to 450 mg/day. Begin dosing at 75 mg two times a day (150 mg/day). The dose may be increased to 150 mg two times a day (300 mg/day) within 1 week based on efficacy and tolerability. Patients who do not experience sufficient benefit with 300 mg/day may be further increased to 225 mg two times a day (450 mg/day). Although LYRICA was also studied at 600 mg/day, there is no evidence that this dose confers additional benefit and this dose was less well tolerated. In view of the dose‑dependent adverse reactions, treatment with doses above 450 mg/day is not recommended
[see Adverse Reactions (6.1)].)2.6 Neuropathic Pain Associated with Spinal Cord Injury in AdultsThe recommended dose range of LYRICA for the treatment of neuropathic pain associated with spinal cord injury is 150 to 600 mg/day. The recommended starting dose is 75 mg two times a day (150 mg/day). The dose may be increased to 150 mg two times a day (300 mg/day) within 1 week based on efficacy and tolerability. Patients who do not experience sufficient pain relief after 2 to 3 weeks of treatment with 150 mg two times a day and who tolerate LYRICA may be treated with up to 300 mg two times a day
[see Clinical Studies (14.5)].• Dosing recommendations:
INDICATION | Dosing Regimen | Maximum Dose | ||||||||||||||||
DPN Pain ( The maximum recommended dose of LYRICA is 100 mg three times a day (300 mg/day) in patients with creatinine clearance of at least 60 mL/min. Begin dosing at 50 mg three times a day (150 mg/day). The dose may be increased to 300 mg/day within 1 week based on efficacy and tolerability. Although LYRICA was also studied at 600 mg/day, there is no evidence that this dose confers additional significant benefit and this dose was less well tolerated. In view of the dose-dependent adverse reactions, treatment with doses above 300 mg/day is not recommended [see Adverse Reactions (6.1)] . | 3 divided doses per day | 300 mg/day within 1 week. | ||||||||||||||||
PHN ( The recommended dose of LYRICA is 75 to 150 mg two times a day, or 50 to 100 mg three times a day (150 to 300 mg/day) in patients with creatinine clearance of at least 60 mL/min. Begin dosing at 75 mg two times a day, or 50 mg three times a day (150 mg/day). The dose may be increased to 300 mg/day within 1 week based on efficacy and tolerability. Patients who do not experience sufficient pain relief following 2 to 4 weeks of treatment with 300 mg/day, and who are able to tolerate LYRICA, may be treated with up to 300 mg two times a day, or 200 mg three times a day (600 mg/day). In view of the dose-dependent adverse reactions and the higher rate of treatment discontinuation due to adverse reactions, reserve dosing above 300 mg/day for those patients who have on-going pain and are tolerating 300 mg daily [see Adverse Reactions (6.1)] . | 2 or 3 divided doses per day | 300 mg/day within 1 week. Maximum dose of 600 mg/day. | ||||||||||||||||
Adjunctive Therapy for Partial‑Onset Seizures in Pediatric and Adult Patients Weighing 30 kg or More ( The recommended dosages for adults and pediatric patients 1 month of age and older are included in Table 1. Administer the total daily dosage orally in two or three divided doses as indicated in Table 1. In pediatric patients, the recommended dosing regimen is dependent upon body weight. Based on clinical response and tolerability, dosage may be increased, approximately weekly.
Both the efficacy and adverse event profiles of LYRICA have been shown to be dose-related. The effect of dose escalation rate on the tolerability of LYRICA has not been formally studied. The efficacy of adjunctive LYRICA in patients taking gabapentin has not been evaluated in controlled trials. Consequently, dosing recommendations for the use of LYRICA with gabapentin cannot be offered. | 2 or 3 divided doses per day | Maximum dose of 600 mg/day. | ||||||||||||||||
Adjunctive Therapy for Partial‑Onset Seizures in Pediatric Patients Weighing Less than 30 kg ( The recommended dosages for adults and pediatric patients 1 month of age and older are included in Table 1. Administer the total daily dosage orally in two or three divided doses as indicated in Table 1. In pediatric patients, the recommended dosing regimen is dependent upon body weight. Based on clinical response and tolerability, dosage may be increased, approximately weekly.
Both the efficacy and adverse event profiles of LYRICA have been shown to be dose-related. The effect of dose escalation rate on the tolerability of LYRICA has not been formally studied. The efficacy of adjunctive LYRICA in patients taking gabapentin has not been evaluated in controlled trials. Consequently, dosing recommendations for the use of LYRICA with gabapentin cannot be offered. | 1 month to less than 4 years: 3 divided doses per day
4 years and older: 2 or 3 divided doses per day | 14 mg/kg/day. | ||||||||||||||||
Fibromyalgia ( The recommended dose of LYRICA for fibromyalgia is 300 to 450 mg/day. Begin dosing at 75 mg two times a day (150 mg/day). The dose may be increased to 150 mg two times a day (300 mg/day) within 1 week based on efficacy and tolerability. Patients who do not experience sufficient benefit with 300 mg/day may be further increased to 225 mg two times a day (450 mg/day). Although LYRICA was also studied at 600 mg/day, there is no evidence that this dose confers additional benefit and this dose was less well tolerated. In view of the dose‑dependent adverse reactions, treatment with doses above 450 mg/day is not recommended [see Adverse Reactions (6.1)] . | 2 divided doses per day | 300 mg/day within 1 week. Maximum dose of 450 mg/day. | ||||||||||||||||
Neuropathic Pain Associated with Spinal Cord Injury ( The recommended dose range of LYRICA for the treatment of neuropathic pain associated with spinal cord injury is 150 to 600 mg/day. The recommended starting dose is 75 mg two times a day (150 mg/day). The dose may be increased to 150 mg two times a day (300 mg/day) within 1 week based on efficacy and tolerability. Patients who do not experience sufficient pain relief after 2 to 3 weeks of treatment with 150 mg two times a day and who tolerate LYRICA may be treated with up to 300 mg two times a day [see Clinical Studies (14.5)] . | 2 divided doses per day | 300 mg/day within 1 week. Maximum dose of 600 mg/day. |
• Dose should be adjusted in adult patients with reduced renal function. ()2.7 Dosing for Adult Patients with Renal ImpairmentIn view of dose-dependent adverse reactions and since LYRICA is eliminated primarily by renal excretion, adjust the dose in adult patients with reduced renal function. The use of LYRICA in pediatric patients with compromised renal function has not been studied.
Base the dose adjustment in patients with renal impairment on creatinine clearance (CLcr), as indicated in Table 2. To use this dosing table, an estimate of the patient’s CLcr in mL/min is needed. CLcr in mL/min may be estimated from serum creatinine (mg/dL) determination using the Cockcroft and Gault equation:
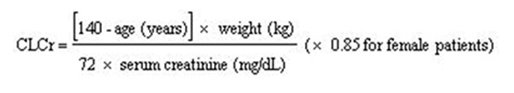
Next, refer to the Dosage and Administration section to determine the recommended total daily dose based on indication, for a patient with normal renal function (CLcr greater than or equal to 60 mL/min). Then refer to Table 2 to determine the corresponding renal adjusted dose.
(For example: A patient initiating LYRICA therapy for postherpetic neuralgia with normal renal function (CLcr greater than or equal to 60 mL/min), receives a total daily dose of 150 mg/day pregabalin. Therefore, a renal impaired patient with a CLcr of 50 mL/min would receive a total daily dose of 75 mg/day pregabalin administered in two or three divided doses.)
For patients undergoing hemodialysis, adjust the pregabalin daily dose based on renal function. In addition to the daily dose adjustment, administer a supplemental dose immediately following every 4-hour hemodialysis treatment (see Table 2).
Table 2. Pregabalin Dosage Adjustment Based on Renal Function TID = Three divided doses; BID = Two divided doses; QD = Single daily dose. Creatinine Clearance (CLcr)(mL/min)Total Pregabalin Daily Dose(mg/day)Total daily dose (mg/day) should be divided as indicated by dose regimen to provide mg/dose.Dose RegimenGreater than or equal to 60
150
300
450
600
BID or TID
30-60
75
150
225
300
BID or TID
15-30
25-50
75
100-150
150
QD or BID
Less than 15
25
25-50
50-75
75
QD
Supplementary dosage following hemodialysis (mg)Supplementary dose is a single additional dose.
Patients on the 25 mg QD regimen: take one supplemental dose of 25 mg or 50 mg
Patients on the 25-50 mg QD regimen: take one supplemental dose of 50 mg or 75 mg
Patients on the 50-75 mg QD regimen: take one supplemental dose of 75 mg or 100 mg
Patients on the 75 mg QD regimen: take one supplemental dose of 100 mg or 150 mg
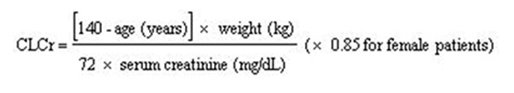
Cockcroft and Gault Equation
Capsules: 25 mg, 50 mg, 75 mg, 100 mg, 150 mg, 200 mg, 225 mg, and 300 mg
Oral Solution: 20 mg/mL
Pregabalin is described chemically as (
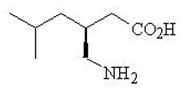
Pregabalin is a white to off-white, crystalline solid with a pKa1of 4.2 and a pKa2of 10.6. It is freely soluble in water and both basic and acidic aqueous solutions. The log of the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is -1.35.
LYRICA (pregabalin) Capsules are administered orally and are supplied as imprinted hard-shell capsules containing 25, 50, 75, 100, 150, 200, 225, and 300 mg of pregabalin, along with cornstarch, lactose monohydrate, and talc as inactive ingredients. The capsule shells contain gelatin and titanium dioxide. In addition, the orange capsule shells contain red iron oxide and the white capsule shells contain colloidal silicon dioxide and sodium lauryl sulfate. Colloidal silicon dioxide is a manufacturing aid that may or may not be present in the capsule shells. The imprinting ink contains black iron oxide, potassium hydroxide, propylene glycol, and shellac.
LYRICA (pregabalin) oral solution, 20 mg/mL, is administered orally and is supplied as a clear, colorless solution contained in a 16 fluid ounce white HDPE bottle with a polyethylene-lined closure. The oral solution contains 20 mg/mL of pregabalin, along with artificial strawberry #11545, dibasic sodium phosphate anhydrous, methylparaben, monobasic sodium phosphate anhydrous, propylparaben, purified water, and sucralose as inactive ingredients.

White, hard-gelatin capsule printed with black ink “VTRS” on the cap, “PGN” over “25” on the body; available in:
Bottles of 90: NDC 58151-236-77
White, hard-gelatin capsule printed with black ink “VTRS” on the cap, “PGN” over “50” and an ink band on the body, available in:
Bottles of 90: NDC 58151-237-77
Unit-Dose Blister Packages of 100: NDC 58151-237-88
White/orange hard gelatin capsule printed with black ink “VTRS” on the cap, “PGN” over “75” on the body; available in:
Bottles of 90: NDC 58151-238-77
Unit-Dose Blister Packages of 100: NDC 58151-238-88
Orange, hard-gelatin capsule printed with black ink “VTRS” on the cap, “PGN” over “100” on the body, available in:
Bottles of 90: NDC 58151-239-77
Unit-Dose Blister Packages of 100: NDC 58151-239-88
White hard gelatin capsule printed with black ink “VTRS” on the cap, “PGN” over “150” on the body, available in:
Bottles of 90: NDC 58151-240-77
Unit-Dose Blister Packages of 100: NDC 58151-240-88
Light orange hard gelatin capsule printed with black ink “VTRS” on the cap, “PGN” over “200” on the body, available in:
Bottles of 90: NDC 58151-241-77
White/light orange hard gelatin capsule printed with black ink “VTRS” on the cap, “PGN” over “225” on the body; available in:
Bottles of 90: NDC 58151-243-77
White/orange hard gelatin capsule printed with black ink “VTRS” on the cap, “PGN” over “300” on the body, available in:
Bottles of 90: NDC 58151-242-77
16 fluid ounce white high density polyethylene (HDPE) bottle with a polyethylene-lined closure:
16 fluid ounce bottle NDC 58151-244-35
Storage and Handling
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) (see USP Controlled Room Temperature).
• Lactation: Breastfeeding is not recommended. ()8.2 LactationRisk SummarySmall amounts of pregabalin have been detected in the milk of lactating women. A pharmacokinetic study in lactating women detected pregabalin in breast milk at average steady state concentrations approximately 76% of those in maternal plasma. The estimated average daily infant dose of pregabalin from breast milk (assuming mean milk consumption of 150 mL/kg/day) was 0.31 mg/kg/day, which on a mg/kg basis would be approximately 7% of the maternal dose
(see Data). The study did not evaluate the effects of LYRICA on milk production or the effects of LYRICA on the breastfed infant.Based on animal studies, there is a potential risk of tumorigenicity with pregabalin exposure via breast milk to the breastfed infant
[see Nonclinical Toxicology (13.1)]. Available clinical study data in patients greater than 12 years of age do not provide a clear conclusion about the potential risk of tumorigenicity with pregabalin[see Warnings and Precautions (5.9)]. Because of the potential risk of tumorigenicity, breastfeeding is not recommended during treatment with LYRICA.DataA pharmacokinetic study in ten lactating women, who were at least 12 weeks postpartum, evaluated the concentrations of pregabalin in plasma and breast milk. LYRICA 150 mg oral capsule was given every 12 hours (300 mg daily dose) for a total of four doses. Pregabalin was detected in breast milk at average steady‑state concentrations approximately 76% of those in maternal plasma. The estimated average daily infant dose of pregabalin from breast milk (assuming mean milk consumption of 150 mL/kg/day) was 0.31 mg/kg/day, which on a mg/kg basis would be approximately 7% of the maternal dose. The study did not evaluate the effects of LYRICA on milk production. Infants did not receive breast milk obtained during the dosing period, therefore, the effects of LYRICA on the breast fed infant were not evaluated.
LYRICA is contraindicated in patients with known hypersensitivity to pregabalin or any of its components. Angioedema and hypersensitivity reactions have occurred in patients receiving pregabalin therapy
There have been postmarketing reports of hypersensitivity in patients shortly after initiation of treatment with LYRICA. Adverse reactions included skin redness, blisters, hives, rash, dyspnea, and wheezing. Discontinue LYRICA immediately in patients with these symptoms.