Meclizine Hydrochloride
Meclizine Hydrochloride Prescribing Information
Meclizine hydrochloride tablet is indicated for the treatment of vertigo associated with diseases affecting the vestibular system in adults.
- 12.5 mg: White to off-white, oval shaped, film-coated tablets, debossed with “CE” on one side and “256” on the other side.
- 25 mg: White to off-white, oval shaped, film-coated tablets, debossed with “CE” on one side and “257” on the other side.
- 50 mg: White to off-white, oval shaped, film-coated scored tablets, debossed with “C” breakline “E” on one side and “258” on the other side.
Meclizine hydrochloride tablets are contraindicated in patients with a hypersensitivity to meclizine or any of the inactive ingredients
The following adverse reactions associated with the use of meclizine hydrochloride tablets were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Anaphylactic reaction, drowsiness, dry mouth, headache, fatigue, and vomiting. On rare occasions blurred vision has been reported.
Common adverse reactions are anaphylactic reaction, drowsiness, dry mouth, headache, fatigue, and vomiting. On rare occasions blurred vision has been reported .
Meclizine Hydrochloride, USP, a histamine (H1) receptor antagonist, is a white or slightly yellowish crystalline powder. It has the following structural formula:
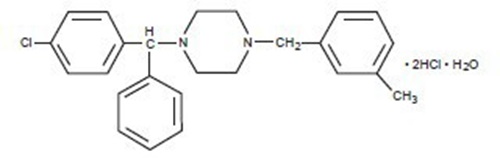
Molecular Weight - 481.88
Chemically, meclizine hydrochloride is 1-(
Inactive ingredients for the tablets are: colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, sodium starch glycolate, stearic acid, talc and titanium dioxide.
Meclizine hydrochloride tablets, USP are available in three strengths, 12.5 mg, 25 mg and 50 mg.
Each meclizine hydrochloride, USP 12.5 mg tablet contains 12.5 mg of meclizine dihydrochloride equivalent to 10.53 mg of meclizine free base.
Each meclizine hydrochloride, USP 25 mg tablet contains 25 mg of meclizine dihydrochloride equivalent to 21.07 mg of meclizine free base.
Each meclizine hydrochloride, USP 50 mg tablet contains 50 mg of meclizine dihydrochloride equivalent to 42.14 mg of meclizine free base.

The following adverse reactions associated with the use of meclizine hydrochloride tablets were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Anaphylactic reaction, drowsiness, dry mouth, headache, fatigue, and vomiting. On rare occasions blurred vision has been reported.
Meclizine Hydrochloride, USP, a histamine (H1) receptor antagonist, is a white or slightly yellowish crystalline powder. It has the following structural formula:

Molecular Weight - 481.88
Chemically, meclizine hydrochloride is 1-(
Inactive ingredients for the tablets are: colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, sodium starch glycolate, stearic acid, talc and titanium dioxide.
Meclizine hydrochloride tablets, USP are available in three strengths, 12.5 mg, 25 mg and 50 mg.
Each meclizine hydrochloride, USP 12.5 mg tablet contains 12.5 mg of meclizine dihydrochloride equivalent to 10.53 mg of meclizine free base.
Each meclizine hydrochloride, USP 25 mg tablet contains 25 mg of meclizine dihydrochloride equivalent to 21.07 mg of meclizine free base.
Each meclizine hydrochloride, USP 50 mg tablet contains 50 mg of meclizine dihydrochloride equivalent to 42.14 mg of meclizine free base.