Megestrol Acetate
Megestrol Acetate Prescribing Information
Megestrol acetate oral suspension is indicated for the treatment of anorexia, cachexia, or an unexplained significant weight loss in patients with a diagnosis of acquired immunodeficiency syndrome (AIDS).
Therapy with megestrol acetate oral suspension for weight loss should only be instituted after treatable causes of weight loss are sought and addressed. These treatable causes include possible malignancies, systemic infections, gastrointestinal disorders affecting absorption, endocrine disease, renal disease or psychiatric diseases.
Megestrol acetate oral suspension is not intended for prophylactic use to avoid weight loss.
Megestrol acetate oral suspension is milky white, lemon flavored, and contains 125 mg per mL.
- History of hypersensitivity to megestrol acetate or any component of the formulation.
- Pregnancy [see Warnings and Precautions (), Use in Specific Populations (
5.2 Fetal ToxicityBased on animal studies, megestrol acetate may cause fetal harm when administered to a pregnant woman. Pregnant rats treated with low doses of megestrol acetate resulted in a reduction in fetal weight and number of live births, and feminization of male fetuses. There are no available human data to assess for any drug associated risks of miscarriage, birth defects, or adverse maternal or fetal outcomes. If this drug is used during pregnancy, or if the patient becomes pregnant while taking (receiving) this drug, advise the patient of the potential hazard to the fetus[see Use in Specific Populations , Nonclinical Toxicology ].Obtain a pregnancy test in females of reproductive potential prior to initiating treatment with megestrol acetate oral suspension[see Dosage and Administration ].Advise females of reproductive potential to use effective contraception while taking megestrol acetate oral suspension[see Use in Specific Populations ].,8.1 PregnancyRisk SummaryBased on animal data, megestrol acetate may cause fetal harm when administered to a pregnant woman and is contraindicated during pregnancy
[see Contraindications ]. There are no available human data to assess for any drug-associated risks of miscarriage, birth defects, or adverse maternal or fetal outcomes. There are no adequate animal developmental toxicity data at clinically relevant doses. Pregnant rats treated with low doses of megestrol acetate resulted in a reduction in fetal weight and number of live births, and feminization of male fetuses at doses below maximum recommended clinical dosing based on body surface area (see Data). Advise a pregnant women of the potential risk to the fetus.The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
DataReproduction studies were performed in pregnant rats at oral doses ranging from 0.05 to 12.5 mg/kg/day, which are below the maximum recommended clinical dose based on body surface area. Reduction in fetal weight and number of live births were observed at 12.5 mg/kg/day (5 times lower than the maximum clinical dose) when dams were dosed on days 12 through 18 of pregnancy. Feminization of male fetuses also occurred when dams were dosed on days 13 through 20 of pregnancy at 3 mg/kg/day, approximately 22 times below the maximum clinical dose.
)].8.3 Females and Males of Reproductive PotentialPregnancy testingPregnancy testing is recommended prior to treatment with megestrol acetate oral suspension
[see Dosage and Administration , Use in Specific Populations ].ContraceptionMegestrol acetate oral suspension may cause fetal harm when administered during pregnancy
[see Use in Specific Populations ].Advise females of reproductive potential to use effective contraception during treatment with megestrol acetate oral suspension.
The most common adverse events occurring in > 5% of all patients receiving 800mg/20mL of megestrol acetate oral suspension in the two clinical efficacy trials were nausea, diarrhea, impotence, rash, flatulence, hypertension, and asthenia
Because clinical trials are conducted under widely varying conditions, adverse reactions observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of megestrol acetate oral suspension, 125 mg/mL was based on three studies of megestrol acetate oral suspension (40 mg/mL). The adverse reaction profile of these 3 studies are presented below.
Adverse events which occurred in at least 5% of patients in any arm of the two clinical efficacy trials and the open trial for megestrol acetate oral suspension are listed below by treatment group. All patients listed had at least one post baseline visit during the 12 study weeks.
Percentage of Patients Reporting Adverse Events | |||||||
Trial 1 (N=236) | Trial 2 (N=87) | Open Label Trial | |||||
Placebo | Placebo | ||||||
Megestrol Acetate mg/day | 0 | 100 | 400 | 800 | 0 | 800 | 1200 |
No. of Patients | N=34 | N=68 | N=69 | N=65 | N=38 | N=49 | N=176 |
Diarrhea | 15 | 13 | 8 | 15 | 8 | 6 | 10 |
Impotence | 3 | 4 | 6 | 14 | 0 | 4 | 7 |
Rash | 9 | 9 | 4 | 12 | 3 | 2 | 6 |
Flatulence | 9 | 0 | 1 | 9 | 3 | 10 | 6 |
Hypertension | 0 | 0 | 0 | 8 | 0 | 0 | 4 |
Asthenia | 3 | 2 | 3 | 6 | 8 | 4 | 5 |
Insomnia | 0 | 3 | 4 | 6 | 0 | 0 | 1 |
Nausea | 9 | 4 | 0 | 5 | 3 | 4 | 5 |
Anemia | 6 | 3 | 3 | 5 | 0 | 0 | 0 |
Fever | 3 | 6 | 4 | 5 | 3 | 2 | 1 |
Libido Decreased | 3 | 4 | 0 | 5 | 0 | 2 | 1 |
Dyspepsia | 0 | 0 | 3 | 3 | 5 | 4 | 2 |
Hyperglycemia | 3 | 0 | 6 | 3 | 0 | 0 | 3 |
Headache | 6 | 10 | 1 | 3 | 3 | 0 | 3 |
Pain | 6 | 0 | 0 | 2 | 5 | 6 | 4 |
Vomiting | 9 | 3 | 0 | 2 | 3 | 6 | 4 |
Pneumonia | 6 | 2 | 0 | 2 | 3 | 0 | 1 |
Urinary Frequency | 0 | 0 | 1 | 2 | 5 | 2 | 1 |
Adverse events which occurred in 1% to 3% of all patients enrolled in the two clinical efficacy trials with at least one follow-up visit during the first 12 weeks of the study are listed below by body system. Adverse events occurring less than 1% are not included. There were no significant differences between incidence of these events in patients treated with megestrol acetate and patients treated with placebo.
Due to a significant decrease in indinavir exposure, administration of a higher dose of indinavir should be considered when coadministering with megestrol acetate (
Due to the significant decrease in the exposure of indinavir by megestrol acetate, administration of a higher dose of indinavir should be considered when coadministering with megestrol acetate
Mean plasma concentrations of megestrol acetate after administration of 625 mg (125 mg/mL) of megestrol acetate oral suspension are equivalent under fed conditions to 800 mg (40 mg/mL) of megestrol acetate oral suspension in healthy volunteers.
In order to characterize the dose proportionality of megestrol acetate oral suspension, pharmacokinetic studies across a range of doses were conducted when administered under fasting and fed conditions. Pharmacokinetics of megestrol acetate was linear in the dosing range between 150 mg and 675 mg after megestrol acetate oral suspension administration regardless of meal condition. The mean peak plasma concentration (Cmax) and the mean area under the concentration time-curve (AUC) after a high fat meal were increased by 48% and 36%, respectively, compared to those under the fasting condition after 625 mg megestrol acetate oral suspension administration. This food effect is less than that seen for the original formulation, megestrol acetate 800 mg/20 mL, where a high fat meal significantly increased AUC and Cmaxof megestrol acetate to 2-fold and 7-fold, respectively, compared to those under the fasting condition. There was no difference in safety following administration in the fed state, therefore megestrol acetate oral suspension could be taken without regard to meals.
Plasma steady state pharmacokinetics of megestrol acetate was evaluated in 10 adult, cachectic male adult patients with acquired immunodeficiency syndrome (AIDS) and an involuntary weight loss greater than 10% of baseline who received single oral doses of 800 mg/day of megestrol acetate oral suspension for 21 days. The Mean (±1SD) Cmaxof megestrol acetate was 753 (±539) ng/mL. The mean AUC was 10476 (±7788) ng x hr/mL. Median Tmaxvalue was five hours.
In another study, 24 asymptomatic HIV seropositive male adult subjects were dosed once daily with 750 mg of megestrol acetate oral suspension for 14 days. Mean Cmaxand AUC values were 490 (±238) ng/mL and 6779 (±3048) hr x ng/mL, respectively. The median Tmaxvalue was three hours. The mean Cminvalue was 202 (±101) ng/mL. The mean % of fluctuation value was 107 (±40).
The major route of drug elimination in humans is urine. When radio-labeled megestrol acetate was administered to humans in doses of 4 to 90 mg, the urinary excretion within 10 days ranged from 56.5% to 78.4% (mean 66.4%) and fecal excretion ranged from 7.7% to 30.3% (mean 19.8%). The total recovered radioactivity varied between 83.1% and 94.7% (mean 86.2%).
Megestrol acetate metabolites which were identified in urine constituted 5% to 8% of the dose administered. Respiratory excretion as labeled carbon dioxide and fat storage may have accounted for at least part of the radioactivity not found in urine and feces.
The mean elimination half-life of megestrol ranged from 20 to 50 hours in healthy subjects.
Specific Populations
The pharmacokinetics of megestrol acetate has not been studied in specific population, for example, pediatric, renal impairment, and hepatic impairment.
The effects of indinavir, zidovudine or rifabutin on the pharmacokinetics of megestrol acetate were not studied.
Zidovudine
Pharmacokinetic studies show that there are no significant alterations in exposure of zidovudine when megestrol acetate is administered with this drug.
Rifabutin
Pharmacokinetic studies show that there are no significant alterations in exposure of rifabutin when megestrol acetate is administered with this drug.
Indinavir
A pharmacokinetic study in healthy male subjects demonstrated that coadministration of megestrol acetate (675 mg for 14 days) and indinavir (single dose 800 mg) results in a significant decrease in the pharmacokinetic parameters (~32% for Cmaxand ~21% for AUC) of indinavir.
Megestrol acetate oral suspension contains megestrol acetate, a synthetic derivative of the naturally occurring steroid hormone, progesterone. Megestrol acetate is a white, crystalline solid chemically designated as 17-Hydroxy-6-methyl pregna-4,6-diene-3,20-dione acetate. Solubility at 37° C in water is 2 mcg per mL, solubility in plasma is 24 mcg per mL. Its molecular weight is 384.52.
The chemical formula is C24H32O4 and the structural formula is represented as follows:
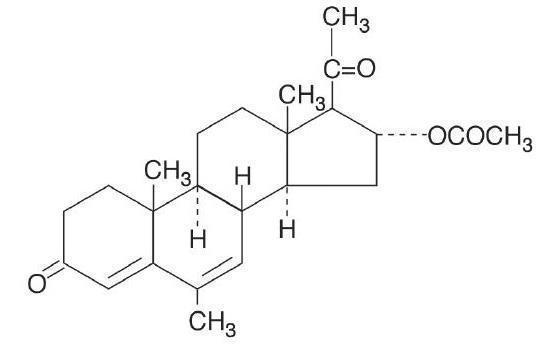
Megestrol acetate oral suspension is an oral suspension containing 125 mg of megestrol acetate per mL.
Megestrol acetate oral suspension contains the following inactive ingredients: alcohol (max 0.07% v/v from flavor), citric acid monohydrate, hypromellose, lemon flavor, sodium benzoate, sodium citrate dihydrate, sodium lauryl sulfate, and sucrose.
The USP dissolution test is pending.