Metaxal
(Metaxalone)Metaxal Prescribing Information
Metaxalone is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomforts associated with acute, painful musculoskeletal conditions. The mode of action of this drug has not been clearly identified, but may be related to its sedative properties. Metaxalone does not directly relax tense skeletal muscles in man.
The recommended dose for adults and children over 12 years of age is two 400 mg tablets (800 mg) three to four times a day.
Known hypersensitivity to any components of this product.
Known tendency to drug induced, hemolytic, or other anemias.
Significantly impaired renal or hepatic function.
Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of serotonergic drugs with metaxalone used within the recommended dosage range (see PRECAUTIONS: Drug Interactions) and with metaxalone as a single agent taken at doses higher than the recommended dose (see OVERDOSAGE). Serotonergic drugs include selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, opioids (particularly fentanyl, meperidine, and methadone), drugs that affect the serotonergic neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), and drugs that impair metabolism of serotonin (including monoamine oxidase (MAO) inhibitors, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue) (see PRECAUTIONS: Drug Interactions).
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination, rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms generally occurs within several hours to a few days, but may occur later than that. Discontinue metaxalone if serotonin syndrome is suspected.
The sedative effects of metaxalone and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants (TCAs) may be additive. Exercise caution with patients who take more than one of these CNS depressants simultaneously. Follow patients closely for signs and symptoms of respiratory depression and sedation (see PRECAUTIONS: Drug Interactions).
The sedative effects of metaxalone and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants (TCAs) may be additive. Exercise caution with patients who take more than one of these CNS depressants simultaneously. Follow patients closely for signs and symptoms of respiratory depression and sedation (see WARNINGS).
Serotonin syndrome has resulted from concomitant use of serotonergic drugs with metaxalone used within the recommended dosage range (see WARNINGS). If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue metaxalone if serotonin syndrome is suspected.
Examples of serotonergic drugs include: selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, opioids (particularly fentanyl, meperidine, and methadone), drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue).
Deaths by deliberate or accidental overdose have occurred with metaxalone, particularly in combination with antidepressants, and have been reported with this class of drug in combination with alcohol.
Serotonin syndrome has been reported when metaxalone was used at doses higher than the recommended dose (see WARNINGSand ADVERSE REACTIONS).
When determining the LD50in rats and mice, progressive sedation, hypnosis and finally respiratory failure were noted as the dosage increased. In dogs, no LD50could be determined as the higher doses produced an emetic action in 15 to 30 minutes.
Gastric lavage and supportive therapy. Consultation with a regional poison control center is recommended.
To report SUSPECTED ADVERSE REACTIONS, contact Trifluent Pharma, LLC at 210-944-6920 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch
The sedative effects of metaxalone and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants (TCAs) may be additive. Exercise caution with patients who take more than one of these CNS depressants simultaneously. Follow patients closely for signs and symptoms of respiratory depression and sedation (see
Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of serotonergic drugs with metaxalone used within the recommended dosage range (see PRECAUTIONS: Drug Interactions) and with metaxalone as a single agent taken at doses higher than the recommended dose (see OVERDOSAGE). Serotonergic drugs include selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, opioids (particularly fentanyl, meperidine, and methadone), drugs that affect the serotonergic neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), and drugs that impair metabolism of serotonin (including monoamine oxidase (MAO) inhibitors, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue) (see PRECAUTIONS: Drug Interactions).
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination, rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms generally occurs within several hours to a few days, but may occur later than that. Discontinue metaxalone if serotonin syndrome is suspected.
The sedative effects of metaxalone and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants (TCAs) may be additive. Exercise caution with patients who take more than one of these CNS depressants simultaneously. Follow patients closely for signs and symptoms of respiratory depression and sedation (see PRECAUTIONS: Drug Interactions).
Metaxalone Tablets, USP are available as 400mg, 400mg tablets are round shaped, light pink tablets.
Chemically, metaxalone, USP is 5-[(3,5-dimethylphenoxy)methyl]-2-oxazolidinone. The empirical formula is C12H15NO3, which corresponds to a molecular weight of 221.25. The structural formula is:
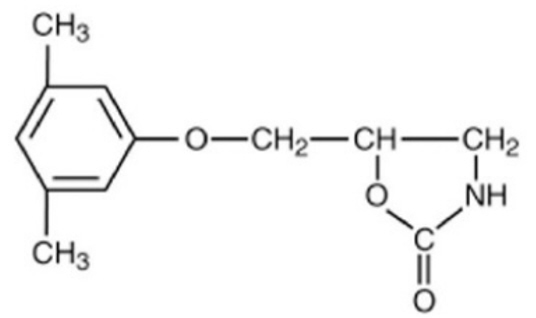
Metaxalone, USP is a white to almost white, crystalline powder freely soluble in dichloromethane, soluble in methanol, sparingly soluble in ethanol and ethyl acetate, slightly soluble in toluene and isopropanol, insoluble in water and n-hexane.
Each tablet contains 400 mg metaxalone, USP and the following inactive ingredients: alginic acid, corn starch, ferric oxide red, copovidone, magnesium stearate, povidone, pregelatinized starch, sodium alginate.