Metformin Hydrochloride Prescribing Information
There have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific symptoms such as malaise, myalgias, abdominal pain, respiratory distress, or increased somnolence; however, hypotension and resistant bradyarrhythmias have occurred with severe acidosis. Metformin-associated lactic acidosis was characterized by elevated blood lactate concentrations (>5 mmol/L), anion gap acidosis (without evidence of ketonuria or ketonemia), and an increased lactate: pyruvate ratio; metformin plasma levels were generally >5 mcg/mL. Metformin decreases liver uptake of lactate increasing lactate blood levels which may increase the risk of lactic acidosis, especially in patients at risk.
If metformin-associated lactic acidosis is suspected, general supportive measures should be instituted promptly in a hospital setting, along with immediate discontinuation of metformin hydrochloride extended-release tablets. In metformin hydrochloride extended-release tablets treated patients with a diagnosis or strong suspicion of lactic acidosis, prompt hemodialysis is recommended to correct the acidosis and remove accumulated metformin (metformin hydrochloride is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions). Hemodialysis has often resulted in reversal of symptoms and recovery.
Educate patients and their families about the symptoms of lactic acidosis and, if these symptoms occur, instruct them to discontinue metformin hydrochloride extended-release tablets and report these symptoms to their healthcare provider.
For each of the known and possible risk factors for metformin-associated lactic acidosis, recommendations to reduce the risk of and manage metformin-associated lactic acidosis are provided below:
- Renal impairment- The postmarketing metformin-associated lactic acidosis cases primarily occurred in patients with significant renal impairment.
The risk of metformin accumulation and metformin-associated lactic acidosis increases with the severity of renal impairment because metformin is substantially excreted by the kidney. Clinical recommendations based upon the patient's renal function include [
- Before initiating metformin hydrochloride extended-release tablets, obtain an estimated glomerular filtration rate (eGFR).
- Metformin hydrochloride extended-release tablets are contraindicated in patients with an eGFR less than 30 mL/min/1.73 m2 [see Contraindications (4)].
- Initiation of metformin hydrochloride extended-release tablets are not recommended in patients with eGFR between 30 to 45 mL/min/1.73 m2.
- Obtain an eGFR at least annually in all patients taking metformin hydrochloride extended-release tablets. In patients at risk for the development of renal impairment (e.g., the elderly), renal function should be assessed more frequently.
- In patients taking metformin hydrochloride extended-release tablets whose eGFR falls below 45 mL/min/1.73 m2, assess the benefit and risk of continuing therapy.
- Drug interactions -The concomitant use of metformin hydrochloride extended-release tablets with specific drugs may increase the risk of metformin-associated lactic acidosis: those that impair renal function, result in significant hemodynamic change, interfere with acid-base balance, or increase metformin accumulation. Consider more frequent monitoring of patients.
- Age 65 or greater -The risk of metformin-associated lactic acidosis increases with the patient's age because elderly patients have a greater likelihood of having hepatic, renal, or cardiac impairment than younger patients. Assess renal function more frequently in elderly patients.
- Radiologic studies with contrast -Administration of intravascular iodinated contrast agents in metformin-treated patients has led to an acute decrease in renal function and the occurrence of lactic acidosis. Stop metformin hydrochloride extended-release tablets at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure, and restart metformin hydrochloride extended-release tablets if renal function is stable.
- Surgery and other procedures- Withholding of food and fluids during surgical or other procedures may increase the risk for volume depletion, hypotension, and renal impairment. Metformin hydrochloride extended-release tablets should be temporarily discontinued while patients have restricted food and fluid intake.
- Hypoxic states- Several of the postmarketing cases of metformin-associated lactic acidosis occurred in the setting of acute congestive heart failure (particularly when accompanied by hypoperfusion and hypoxemia). Cardiovascular collapse (shock), acute myocardial infarction, sepsis, and other conditions associated with hypoxemia have been associated with lactic acidosis and may cause prerenal azotemia. When such an event occurs, discontinue metformin hydrochloride extended-release tablets.
- Excessive alcohol intake -Alcohol potentiates the effect of metformin on lactate metabolism. Patients should be warned against excessive alcohol intake while receiving metformin hydrochloride extended-release tablets.
- Hepatic impairment -Patients with hepatic impairment have developed cases of metformin-associated lactic acidosis. This may be due to impaired lactate clearance resulting in higher lactate blood levels. Therefore, avoid use of metformin hydrochloride extended-release tablets in patients with clinical or laboratory evidence of hepatic disease.
Discontinue metformin hydrochloride extended-release tablets at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of liver disease, alcoholism, or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure; restart metformin hydrochloride extended-release tablets if renal function is stable.
Metformin hydrochloride extended-release tablets are contraindicated in patients with:
- Severe renal impairment (eGFR below 30 mL/min/1.73 m2) [see Warnings and Precautions (5.1)].
- Hypersensitivity to metformin.
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma.
- Severe renal impairment (eGFR below 30 mL/min/1.73 m2)
- Hypersensitivity to metformin
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma.
There have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific symptoms such as malaise, myalgias, abdominal pain, respiratory distress, or increased somnolence; however, hypotension and resistant bradyarrhythmias have occurred with severe acidosis. Metformin-associated lactic acidosis was characterized by elevated blood lactate concentrations (>5 mmol/L), anion gap acidosis (without evidence of ketonuria or ketonemia), and an increased lactate: pyruvate ratio; metformin plasma levels were generally >5 mcg/mL. Metformin decreases liver uptake of lactate increasing lactate blood levels which may increase the risk of lactic acidosis, especially in patients at risk.
If metformin-associated lactic acidosis is suspected, general supportive measures should be instituted promptly in a hospital setting, along with immediate discontinuation of metformin hydrochloride extended-release tablets. In metformin hydrochloride extended-release tablets treated patients with a diagnosis or strong suspicion of lactic acidosis, prompt hemodialysis is recommended to correct the acidosis and remove accumulated metformin (metformin hydrochloride is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions). Hemodialysis has often resulted in reversal of symptoms and recovery.
Educate patients and their families about the symptoms of lactic acidosis and, if these symptoms occur, instruct them to discontinue metformin hydrochloride extended-release tablets and report these symptoms to their healthcare provider.
For each of the known and possible risk factors for metformin-associated lactic acidosis, recommendations to reduce the risk of and manage metformin-associated lactic acidosis are provided below:
- Renal impairment- The postmarketing metformin-associated lactic acidosis cases primarily occurred in patients with significant renal impairment.
The risk of metformin accumulation and metformin-associated lactic acidosis increases with the severity of renal impairment because metformin is substantially excreted by the kidney. Clinical recommendations based upon the patient's renal function include [
- Before initiating metformin hydrochloride extended-release tablets, obtain an estimated glomerular filtration rate (eGFR).
- Metformin hydrochloride extended-release tablets are contraindicated in patients with an eGFR less than 30 mL/min/1.73 m2 [see Contraindications (4)].
- Initiation of metformin hydrochloride extended-release tablets are not recommended in patients with eGFR between 30 to 45 mL/min/1.73 m2.
- Obtain an eGFR at least annually in all patients taking metformin hydrochloride extended-release tablets. In patients at risk for the development of renal impairment (e.g., the elderly), renal function should be assessed more frequently.
- In patients taking metformin hydrochloride extended-release tablets whose eGFR falls below 45 mL/min/1.73 m2, assess the benefit and risk of continuing therapy.
- Drug interactions -The concomitant use of metformin hydrochloride extended-release tablets with specific drugs may increase the risk of metformin-associated lactic acidosis: those that impair renal function, result in significant hemodynamic change, interfere with acid-base balance, or increase metformin accumulation. Consider more frequent monitoring of patients.
- Age 65 or greater -The risk of metformin-associated lactic acidosis increases with the patient's age because elderly patients have a greater likelihood of having hepatic, renal, or cardiac impairment than younger patients. Assess renal function more frequently in elderly patients.
- Radiologic studies with contrast -Administration of intravascular iodinated contrast agents in metformin-treated patients has led to an acute decrease in renal function and the occurrence of lactic acidosis. Stop metformin hydrochloride extended-release tablets at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure, and restart metformin hydrochloride extended-release tablets if renal function is stable.
- Surgery and other procedures- Withholding of food and fluids during surgical or other procedures may increase the risk for volume depletion, hypotension, and renal impairment. Metformin hydrochloride extended-release tablets should be temporarily discontinued while patients have restricted food and fluid intake.
- Hypoxic states- Several of the postmarketing cases of metformin-associated lactic acidosis occurred in the setting of acute congestive heart failure (particularly when accompanied by hypoperfusion and hypoxemia). Cardiovascular collapse (shock), acute myocardial infarction, sepsis, and other conditions associated with hypoxemia have been associated with lactic acidosis and may cause prerenal azotemia. When such an event occurs, discontinue metformin hydrochloride extended-release tablets.
- Excessive alcohol intake -Alcohol potentiates the effect of metformin on lactate metabolism. Patients should be warned against excessive alcohol intake while receiving metformin hydrochloride extended-release tablets.
- Hepatic impairment -Patients with hepatic impairment have developed cases of metformin-associated lactic acidosis. This may be due to impaired lactate clearance resulting in higher lactate blood levels. Therefore, avoid use of metformin hydrochloride extended-release tablets in patients with clinical or laboratory evidence of hepatic disease.
There have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific symptoms such as malaise, myalgias, abdominal pain, respiratory distress, or increased somnolence; however, hypotension and resistant bradyarrhythmias have occurred with severe acidosis. Metformin-associated lactic acidosis was characterized by elevated blood lactate concentrations (>5 mmol/L), anion gap acidosis (without evidence of ketonuria or ketonemia), and an increased lactate: pyruvate ratio; metformin plasma levels were generally >5 mcg/mL. Metformin decreases liver uptake of lactate increasing lactate blood levels which may increase the risk of lactic acidosis, especially in patients at risk.
If metformin-associated lactic acidosis is suspected, general supportive measures should be instituted promptly in a hospital setting, along with immediate discontinuation of metformin hydrochloride extended-release tablets. In metformin hydrochloride extended-release tablets treated patients with a diagnosis or strong suspicion of lactic acidosis, prompt hemodialysis is recommended to correct the acidosis and remove accumulated metformin (metformin hydrochloride is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions). Hemodialysis has often resulted in reversal of symptoms and recovery.
Educate patients and their families about the symptoms of lactic acidosis and, if these symptoms occur, instruct them to discontinue metformin hydrochloride extended-release tablets and report these symptoms to their healthcare provider.
For each of the known and possible risk factors for metformin-associated lactic acidosis, recommendations to reduce the risk of and manage metformin-associated lactic acidosis are provided below:
- Renal impairment- The postmarketing metformin-associated lactic acidosis cases primarily occurred in patients with significant renal impairment.
The risk of metformin accumulation and metformin-associated lactic acidosis increases with the severity of renal impairment because metformin is substantially excreted by the kidney. Clinical recommendations based upon the patient's renal function include [
- Before initiating metformin hydrochloride extended-release tablets, obtain an estimated glomerular filtration rate (eGFR).
- Metformin hydrochloride extended-release tablets are contraindicated in patients with an eGFR less than 30 mL/min/1.73 m2 [see Contraindications (4)].
- Initiation of metformin hydrochloride extended-release tablets are not recommended in patients with eGFR between 30 to 45 mL/min/1.73 m2.
- Obtain an eGFR at least annually in all patients taking metformin hydrochloride extended-release tablets. In patients at risk for the development of renal impairment (e.g., the elderly), renal function should be assessed more frequently.
- In patients taking metformin hydrochloride extended-release tablets whose eGFR falls below 45 mL/min/1.73 m2, assess the benefit and risk of continuing therapy.
- Drug interactions -The concomitant use of metformin hydrochloride extended-release tablets with specific drugs may increase the risk of metformin-associated lactic acidosis: those that impair renal function, result in significant hemodynamic change, interfere with acid-base balance, or increase metformin accumulation. Consider more frequent monitoring of patients.
- Age 65 or greater -The risk of metformin-associated lactic acidosis increases with the patient's age because elderly patients have a greater likelihood of having hepatic, renal, or cardiac impairment than younger patients. Assess renal function more frequently in elderly patients.
- Radiologic studies with contrast -Administration of intravascular iodinated contrast agents in metformin-treated patients has led to an acute decrease in renal function and the occurrence of lactic acidosis. Stop metformin hydrochloride extended-release tablets at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure, and restart metformin hydrochloride extended-release tablets if renal function is stable.
- Surgery and other procedures- Withholding of food and fluids during surgical or other procedures may increase the risk for volume depletion, hypotension, and renal impairment. Metformin hydrochloride extended-release tablets should be temporarily discontinued while patients have restricted food and fluid intake.
- Hypoxic states- Several of the postmarketing cases of metformin-associated lactic acidosis occurred in the setting of acute congestive heart failure (particularly when accompanied by hypoperfusion and hypoxemia). Cardiovascular collapse (shock), acute myocardial infarction, sepsis, and other conditions associated with hypoxemia have been associated with lactic acidosis and may cause prerenal azotemia. When such an event occurs, discontinue metformin hydrochloride extended-release tablets.
- Excessive alcohol intake -Alcohol potentiates the effect of metformin on lactate metabolism. Patients should be warned against excessive alcohol intake while receiving metformin hydrochloride extended-release tablets.
- Hepatic impairment -Patients with hepatic impairment have developed cases of metformin-associated lactic acidosis. This may be due to impaired lactate clearance resulting in higher lactate blood levels. Therefore, avoid use of metformin hydrochloride extended-release tablets in patients with clinical or laboratory evidence of hepatic disease.
Metformin hydrochloride extended-release tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
- Swallow metformin hydrochloride extended-release tablets whole and never crush, cut or chew)
2.1 Adult DosageMetformin Hydrochloride Extended-Release Tablets:- Swallow metformin hydrochloride extended-release tablets whole and never crush, cut or chew.
- The recommended starting dose of metformin hydrochloride extended-release tablets is 500 mg orally once daily with the evening meal.
- Increase the dose in increments of 500 mg weekly on the basis of glycemic control and tolerability, up to a maximum of 2000 mg once daily with the evening meal.
- If glycemic control is not achieved with metformin hydrochloride extended-release tablets 2000 mg once daily, consider a trial of metformin hydrochloride extended-release tablets 1000 mg twice daily. If higher doses are required, switch to metformin hydrochloride tablets at total daily doses up to 2550 mg administered in divided daily doses, as described above.
- Patients receiving metformin hydrochloride tablets may be switched to metformin hydrochloride extended-release tablets once daily at the same total daily dose, up to 2000 mg once daily.
- Starting dose: 500 mg orally once daily with the evening meal ()
2.1 Adult DosageMetformin Hydrochloride Extended-Release Tablets:- Swallow metformin hydrochloride extended-release tablets whole and never crush, cut or chew.
- The recommended starting dose of metformin hydrochloride extended-release tablets is 500 mg orally once daily with the evening meal.
- Increase the dose in increments of 500 mg weekly on the basis of glycemic control and tolerability, up to a maximum of 2000 mg once daily with the evening meal.
- If glycemic control is not achieved with metformin hydrochloride extended-release tablets 2000 mg once daily, consider a trial of metformin hydrochloride extended-release tablets 1000 mg twice daily. If higher doses are required, switch to metformin hydrochloride tablets at total daily doses up to 2550 mg administered in divided daily doses, as described above.
- Patients receiving metformin hydrochloride tablets may be switched to metformin hydrochloride extended-release tablets once daily at the same total daily dose, up to 2000 mg once daily.
- Increase the dose in increments of 500 mg weekly, up to a maximum of 2000 mg once daily with the evening meal ()
2.1 Adult DosageMetformin Hydrochloride Extended-Release Tablets:- Swallow metformin hydrochloride extended-release tablets whole and never crush, cut or chew.
- The recommended starting dose of metformin hydrochloride extended-release tablets is 500 mg orally once daily with the evening meal.
- Increase the dose in increments of 500 mg weekly on the basis of glycemic control and tolerability, up to a maximum of 2000 mg once daily with the evening meal.
- If glycemic control is not achieved with metformin hydrochloride extended-release tablets 2000 mg once daily, consider a trial of metformin hydrochloride extended-release tablets 1000 mg twice daily. If higher doses are required, switch to metformin hydrochloride tablets at total daily doses up to 2550 mg administered in divided daily doses, as described above.
- Patients receiving metformin hydrochloride tablets may be switched to metformin hydrochloride extended-release tablets once daily at the same total daily dose, up to 2000 mg once daily.
- Patients receiving metformin hydrochloride tablets may be switched to metformin hydrochloride extended-release tablets once daily at the same total daily dose, up to 2000 mg once daily ()
2.1 Adult DosageMetformin Hydrochloride Extended-Release Tablets:- Swallow metformin hydrochloride extended-release tablets whole and never crush, cut or chew.
- The recommended starting dose of metformin hydrochloride extended-release tablets is 500 mg orally once daily with the evening meal.
- Increase the dose in increments of 500 mg weekly on the basis of glycemic control and tolerability, up to a maximum of 2000 mg once daily with the evening meal.
- If glycemic control is not achieved with metformin hydrochloride extended-release tablets 2000 mg once daily, consider a trial of metformin hydrochloride extended-release tablets 1000 mg twice daily. If higher doses are required, switch to metformin hydrochloride tablets at total daily doses up to 2550 mg administered in divided daily doses, as described above.
- Patients receiving metformin hydrochloride tablets may be switched to metformin hydrochloride extended-release tablets once daily at the same total daily dose, up to 2000 mg once daily.
- Prior to initiation, assess renal function with estimated glomerular filtration rate (eGFR) ()2.3 Recommendations for Use in Renal Impairment
- Assess renal function prior to initiation of metformin hydrochloride extended-release tablets and periodically thereafter.
- Metformin hydrochloride extended-release tablets are contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/minute/1.73 m2.
- Initiation of metformin hydrochloride extended-release tablets in patients with an eGFR between 30 to 45 mL/minute/1.73 m2is not recommended.
- In patients taking metformin hydrochloride extended-release tablets whose eGFR later falls below 45 mL/min/1.73 m2, assess the benefit risk of continuing therapy.
- Discontinue metformin hydrochloride extended-release tablets if the patient's eGFR later falls below 30 mL/minute/1.73 m2[see Warnings and Precautions (5.1)].
- Do not use in patients with eGFR below 30 mL/minute/1.73 m
2()2.3 Recommendations for Use in Renal Impairment- Assess renal function prior to initiation of metformin hydrochloride extended-release tablets and periodically thereafter.
- Metformin hydrochloride extended-release tablets are contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/minute/1.73 m2.
- Initiation of metformin hydrochloride extended-release tablets in patients with an eGFR between 30 to 45 mL/minute/1.73 m2is not recommended.
- In patients taking metformin hydrochloride extended-release tablets whose eGFR later falls below 45 mL/min/1.73 m2, assess the benefit risk of continuing therapy.
- Discontinue metformin hydrochloride extended-release tablets if the patient's eGFR later falls below 30 mL/minute/1.73 m2[see Warnings and Precautions (5.1)].
- Initiation is not recommended in patients with eGFR between 30 to 45 mL/minute/1.73 m
2()2.3 Recommendations for Use in Renal Impairment- Assess renal function prior to initiation of metformin hydrochloride extended-release tablets and periodically thereafter.
- Metformin hydrochloride extended-release tablets are contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/minute/1.73 m2.
- Initiation of metformin hydrochloride extended-release tablets in patients with an eGFR between 30 to 45 mL/minute/1.73 m2is not recommended.
- In patients taking metformin hydrochloride extended-release tablets whose eGFR later falls below 45 mL/min/1.73 m2, assess the benefit risk of continuing therapy.
- Discontinue metformin hydrochloride extended-release tablets if the patient's eGFR later falls below 30 mL/minute/1.73 m2[see Warnings and Precautions (5.1)].
- Assess risk/benefit of continuing if eGFR falls below 45 mL/ minute/1.73 m
2(2.3 Recommendations for Use in Renal Impairment- Assess renal function prior to initiation of metformin hydrochloride extended-release tablets and periodically thereafter.
- Metformin hydrochloride extended-release tablets are contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/minute/1.73 m2.
- Initiation of metformin hydrochloride extended-release tablets in patients with an eGFR between 30 to 45 mL/minute/1.73 m2is not recommended.
- In patients taking metformin hydrochloride extended-release tablets whose eGFR later falls below 45 mL/min/1.73 m2, assess the benefit risk of continuing therapy.
- Discontinue metformin hydrochloride extended-release tablets if the patient's eGFR later falls below 30 mL/minute/1.73 m2[see Warnings and Precautions (5.1)].
- Discontinue if eGFR falls below 30 mL/minute/1.73 m
2()2.3 Recommendations for Use in Renal Impairment- Assess renal function prior to initiation of metformin hydrochloride extended-release tablets and periodically thereafter.
- Metformin hydrochloride extended-release tablets are contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/minute/1.73 m2.
- Initiation of metformin hydrochloride extended-release tablets in patients with an eGFR between 30 to 45 mL/minute/1.73 m2is not recommended.
- In patients taking metformin hydrochloride extended-release tablets whose eGFR later falls below 45 mL/min/1.73 m2, assess the benefit risk of continuing therapy.
- Discontinue metformin hydrochloride extended-release tablets if the patient's eGFR later falls below 30 mL/minute/1.73 m2[see Warnings and Precautions (5.1)].
- Metformin hydrochloride extended-release tablets may need to be discontinued at time of, or prior to, iodinated contrast imaging procedures ()2.4 Discontinuation for Iodinated Contrast Imaging Procedures
Discontinue metformin hydrochloride extended-release tablets at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of liver disease, alcoholism, or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure; restart metformin hydrochloride extended-release tablets if renal function is stable.
Metformin hydrochloride extended-release tablets USP, 500 mg are available as white to off white capsule shaped, biconvex, beveled edge tablet, with occasionally mottled appearance, debossed with "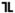 001" on one side and plain on other side.
001" on one side and plain on other side.
- Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy. ()8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as therapy with metformin hydrochloride extended-release tablets may result in ovulation in some anovulatory women.
- Geriatric Use: Assess renal function more frequently. ()
8.5 Geriatric UseControlled clinical studies of metformin hydrochloride extended-release tablets did not include sufficient numbers of elderly patients to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy and the higher risk of lactic acidosis. Assess renal function more frequently in elderly patients [
see Warnings and Precautions (5.1)]. - Hepatic Impairment: Avoid use in patients with hepatic impairment. ()
8 USE IN SPECIFIC POPULATIONS- Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy.
- Geriatric Use: Assess renal function more frequently.
- Hepatic Impairment: Avoid use in patients with hepatic impairment.
8.1 PregnancyRisk SummaryLimited data with metformin hydrochloride extended-release tablets in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or miscarriage. Published studies with metformin use during pregnancy have not reported a clear association with metformin and major birth defect or miscarriage risk
[see Data].There are risks to the mother and fetus associated with poorly controlled diabetes mellitus in pregnancy[see Clinical Considerations].No adverse developmental effects were observed when metformin was administered to pregnant Sprague Dawley rats and rabbits during the period of organogenesis at doses up to 2- and 5 times, respectively, a 2550 mg clinical dose, based on body surface area
[see Data].The estimated background risk of major birth defects is 6 to 10% in women with pre-gestational diabetes mellitus with an HbA1C >7 and has been reported to be as high as 20 to 25% in women with a HbA1C >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical ConsiderationsDisease-associated maternal and/or embryo/fetal riskPoorly-controlled diabetes mellitus in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, stillbirth and delivery complications. Poorly controlled diabetes mellitus increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
DataHuman DataPublished data from post-marketing studies have not reported a clear association with metformin and major birth defects, miscarriage, or adverse maternal or fetal outcomes when metformin was used during pregnancy. However, these studies cannot definitely establish the absence of any metformin-associated risk because of methodological limitations, including small sample size and inconsistent comparator groups.
Animal DataMetformin hydrochloride did not adversely affect development outcomes when administered to pregnant rats and rabbits at doses up to 600 mg/kg/day. This represents an exposure of about 2 and 5 times a 2550 mg clinical dose based on body surface area comparisons for rats and rabbits, respectively. Determination of fetal concentrations demonstrated a partial placental barrier to metformin.
8.2 LactationRisk SummaryLimited published studies report that metformin is present in human milk
[see Data].However, there is insufficient information to determine the effects of metformin on the breastfed infant and no available information on the effects of metformin on milk production. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for metformin hydrochloride extended-release tablets and any potential adverse effects on the breastfed child from metformin hydrochloride extended-release tablets or from the underlying maternal condition.DataPublished clinical lactation studies report that metformin is present in human milk which resulted in infant doses approximately 0.11% to 1% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 0.13 and 1. However, the studies were not designed to definitely establish the risk of use of metformin during lactation because of small sample size and limited adverse event data collected in infants.
8.3 Females and Males of Reproductive PotentialDiscuss the potential for unintended pregnancy with premenopausal women as therapy with metformin hydrochloride extended-release tablets may result in ovulation in some anovulatory women.
8.4 Pediatric UseMetformin Hydrochloride Extended-Release Tablets:Safety and effectiveness of metformin hydrochloride extended-release tablets in pediatric patients have not been established.
8.5 Geriatric UseControlled clinical studies of metformin hydrochloride extended-release tablets did not include sufficient numbers of elderly patients to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy and the higher risk of lactic acidosis. Assess renal function more frequently in elderly patients [
see Warnings and Precautions (5.1)].8.6 Renal ImpairmentMetformin is substantially excreted by the kidney, and the risk of metformin accumulation and lactic acidosis increases with the degree of renal impairment. Metformin hydrochloride extended-release tablets are contraindicated in severe renal impairment, patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2[
see Dosage and Administration (2.3), Contraindications (4), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].8.7 Hepatic ImpairmentUse of metformin in patients with hepatic impairment has been associated with some cases of lactic acidosis. Metformin hydrochloride extended-release tablets are not recommended in patients with hepatic impairment.
[see Warnings and Precautions (5.1)].
Metformin hydrochloride extended-release tablets are contraindicated in patients with:
- Severe renal impairment (eGFR below 30 mL/min/1.73 m
2) [].5.1 Lactic AcidosisThere have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific symptoms such as malaise, myalgias, abdominal pain, respiratory distress, or increased somnolence; however, hypotension and resistant bradyarrhythmias have occurred with severe acidosis. Metformin-associated lactic acidosis was characterized by elevated blood lactate concentrations (>5 mmol/L), anion gap acidosis (without evidence of ketonuria or ketonemia), and an increased lactate: pyruvate ratio; metformin plasma levels were generally >5 mcg/mL. Metformin decreases liver uptake of lactate increasing lactate blood levels which may increase the risk of lactic acidosis, especially in patients at risk.
If metformin-associated lactic acidosis is suspected, general supportive measures should be instituted promptly in a hospital setting, along with immediate discontinuation of metformin hydrochloride extended-release tablets. In metformin hydrochloride extended-release tablets treated patients with a diagnosis or strong suspicion of lactic acidosis, prompt hemodialysis is recommended to correct the acidosis and remove accumulated metformin (metformin hydrochloride is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions). Hemodialysis has often resulted in reversal of symptoms and recovery.
Educate patients and their families about the symptoms of lactic acidosis and, if these symptoms occur, instruct them to discontinue metformin hydrochloride extended-release tablets and report these symptoms to their healthcare provider.
For each of the known and possible risk factors for metformin-associated lactic acidosis, recommendations to reduce the risk of and manage metformin-associated lactic acidosis are provided below:
- Renal impairment- The postmarketing metformin-associated lactic acidosis cases primarily occurred in patients with significant renal impairment.
The risk of metformin accumulation and metformin-associated lactic acidosis increases with the severity of renal impairment because metformin is substantially excreted by the kidney. Clinical recommendations based upon the patient's renal function include [
see Dosage and Administration (2.1),Clinical Pharmacology (12.3)]:- Before initiating metformin hydrochloride extended-release tablets, obtain an estimated glomerular filtration rate (eGFR).
- Metformin hydrochloride extended-release tablets are contraindicated in patients with an eGFR less than 30 mL/min/1.73 m2 [see Contraindications (4)].
- Initiation of metformin hydrochloride extended-release tablets are not recommended in patients with eGFR between 30 to 45 mL/min/1.73 m2.
- Obtain an eGFR at least annually in all patients taking metformin hydrochloride extended-release tablets. In patients at risk for the development of renal impairment (e.g., the elderly), renal function should be assessed more frequently.
- In patients taking metformin hydrochloride extended-release tablets whose eGFR falls below 45 mL/min/1.73 m2, assess the benefit and risk of continuing therapy.
- Drug interactions -The concomitant use of metformin hydrochloride extended-release tablets with specific drugs may increase the risk of metformin-associated lactic acidosis: those that impair renal function, result in significant hemodynamic change, interfere with acid-base balance, or increase metformin accumulation. Consider more frequent monitoring of patients.
- Age 65 or greater -The risk of metformin-associated lactic acidosis increases with the patient's age because elderly patients have a greater likelihood of having hepatic, renal, or cardiac impairment than younger patients. Assess renal function more frequently in elderly patients.
- Radiologic studies with contrast -Administration of intravascular iodinated contrast agents in metformin-treated patients has led to an acute decrease in renal function and the occurrence of lactic acidosis. Stop metformin hydrochloride extended-release tablets at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure, and restart metformin hydrochloride extended-release tablets if renal function is stable.
- Surgery and other procedures- Withholding of food and fluids during surgical or other procedures may increase the risk for volume depletion, hypotension, and renal impairment. Metformin hydrochloride extended-release tablets should be temporarily discontinued while patients have restricted food and fluid intake.
- Hypoxic states- Several of the postmarketing cases of metformin-associated lactic acidosis occurred in the setting of acute congestive heart failure (particularly when accompanied by hypoperfusion and hypoxemia). Cardiovascular collapse (shock), acute myocardial infarction, sepsis, and other conditions associated with hypoxemia have been associated with lactic acidosis and may cause prerenal azotemia. When such an event occurs, discontinue metformin hydrochloride extended-release tablets.
- Excessive alcohol intake -Alcohol potentiates the effect of metformin on lactate metabolism. Patients should be warned against excessive alcohol intake while receiving metformin hydrochloride extended-release tablets.
- Hepatic impairment -Patients with hepatic impairment have developed cases of metformin-associated lactic acidosis. This may be due to impaired lactate clearance resulting in higher lactate blood levels. Therefore, avoid use of metformin hydrochloride extended-release tablets in patients with clinical or laboratory evidence of hepatic disease.
- Hypersensitivity to metformin.
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma.