Methylphenidate Transdermal System Prescribing Information
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:
- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
Consider the possibility of multiple drug ingestion. Because methylphenidate has a large volume of distribution and is rapidly metabolized, dialysis is not useful. Remove all transdermal systems immediately and cleanse the area(s) to remove any remaining adhesive. The continuing absorption of methylphenidate from the skin, even after removal of the transdermal system, should be considered when treating patients with overdose.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
Methylphenidate Transdermal System has a high potential for abuse and misuse. The use of Methylphenidate Transdermal System exposes individuals to the risks of abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Methylphenidate Transdermal System can be diverted for non-medical use into illicit channels or distribution
Before prescribing Methylphenidate Transdermal System, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their caregivers or families about these risks. Advise patients to store Methylphenidate Transdermal System in a safe place, preferably locked, and instruct patients to not give Methylphenidate Transdermal System to anyone else. Throughout Methylphenidate Transdermal System treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
Methylphenidate Transdermal System has special disposal instructions. Instruct patients to find a take back location to dispose of unused or expired Methylphenidate Transdermal System. If a take back program is unavailable, instruct them to:
- Remove Methylphenidate Transdermal System from its pouch, separate it from its liner, fold it in half with the adhesive sides touching each other, and immediately flush the used transdermal system down the toilet, and
- Place the pouch and liner in a container, close the container, and throw out the container in the trash (advise patients not to flush the pouch and liner down the toilet).
Methylphenidate Transdermal System has a high potential for abuse and misuse which can lead to the development of a substance use disorder, including addiction
Abuse is the intentional non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.
Misuse and abuse of methylphenidate may cause increased heart rate, respiratory rate, or blood pressure; sweating; dilated pupils; hyperactivity; restlessness; insomnia; decreased appetite; loss of coordination; tremors; flushed skin; vomiting; and/or abdominal pain. Anxiety, psychosis, hostility, aggression, and suicidal or homicidal ideation have also been observed with CNS stimulants abuse and/or misuse. Misuse and abuse of CNS stimulants, including Methylphenidate Transdermal System, can result in overdose and death
Methylphenidate Transdermal System (methylphenidate transdermal system) is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in pediatric patients 6 to 17 years of age.
The use of Methylphenidate Transdermal System is not recommended in pediatric patients younger than 6 years of age because they had higher plasma exposure and a higher incidence of adverse reactions (e.g., weight loss) than patients 6 years and older at the same dosage
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated children over 36 months (to the ages of 10 to 13 years), suggests that pediatric patients who received methylphenidate for 7 days per week throughout the year had a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this development period.
Closely monitor growth (weight and height) in Methylphenidate Transdermal System -treated pediatric patients. Pediatric patients not growing or gaining height or weight as expected may need to have their treatment interrupted.
The safety and effectiveness of Methylphenidate Transdermal System have not been established in pediatric patients below the age of 6 years.
In studies evaluating extended-release methylphenidate products, patients 4 to <6 years of age had higher systemic methylphenidate exposures than those observed in older pediatric patients at the same dosage. Pediatric patients 4 to <6 years of age also had a higher incidence of adverse reactions, including weight loss.
The safety and effectiveness of Methylphenidate Transdermal System for the treatment of ADHD have been established in pediatric patients 6 to 17 years.
Growth should be monitored during treatment with stimulants, including Methylphenidate Transdermal System. Children who are not growing or gaining weight as expected may need to have their treatment interrupted
Rats treated with methylphenidate early in the postnatal period through sexual maturation demonstrated a decrease in spontaneous locomotor activity in adulthood. A deficit in acquisition of a specific learning task was observed in females only.
Studies with transdermal methylphenidate have not been performed in juvenile animals. In a study conducted in young rats, methylphenidate was administered orally at doses of up to 100 mg/kg/day for 9 weeks, starting early in the postnatal period (Postnatal Day 7) and continuing through sexual maturity (Postnatal Week 10). When these animals were tested as adults (Postnatal Weeks 13-14), decreased spontaneous locomotor activity was observed in males and females previously treated with 50 mg/kg/day or greater, and a deficit in the acquisition of a specific learning task was seen in females exposed to the highest dose. The no effect level for juvenile neurobehavioral development in rats was 5 mg/kg/day. The clinical significance of the long-term behavioral effects observed in rats is unknown.
Four dosage strengths are available:
*Nominal in vivo delivery rate in children and adolescents when applied to the hip, based on a 9-hour wear period. | |||||
Nominal Dose Delivered (mg) Over 9 Hours* | Dosage Rate* (mg/hr) | Transdermal System Size (cm2) | Methylphenidate Content per Transdermal System (mg) | ||
| 10 | 1.1 | 12.5 | 27.5 | ||
| 15 | 1.6 | 18.75 | 41.3 | ||
| 20 | 2.2 | 25 | 55 | ||
30 | 3.3 | 37.5 | 82.5 | ||
Detailed information on serious and adverse reactions of particular importance is provided in the
WARNING: ABUSE, MISUSE, AND ADDICTION
- Before prescribing Methylphenidate Transdermal System, assess each patient’s risk for abuse,misuse, and addiction.
- Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug.
- Throughout treatment, reassess each patient’s risk and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
- Risks to Patients with Serious Cardiac Disease: Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmias, coronary artery disease, or other serious cardiac disease.
- Increased Blood Pressure and Heart Rate: Monitor blood pressure and pulse.
- Psychiatric Adverse Reactions: Prior to initiating Methylphenidate Transdermal System, screen patients for risk factors for developing a manic episode. If new psychotic or manic symptoms occur, consider discontinuing Methylphenidate Transdermal System.
- Seizures: Stimulants may lower the convulsive threshold. Discontinue in the presence of seizures.
- Priapism: If abnormally sustained or frequent and painful erections occur, patients should seek immediate medical attention.
- Peripheral Vasculopathy, including Raynaud’s phenomenon: Careful observation for digital changes is necessary during Methylphenidate Transdermal System treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for patients who develop signs or symptoms of peripheral vasculopathy.
- Long-Term Suppression of Growth in Pediatric Patients: Closely monitor (height and weight) in pediatric patients. Pediatric patients not growing or gaining height or weight as expected may need to have their treatment interrupted.
- Chemical Leukoderma: Methylphenidate Transdermal System use may result in a persistent loss of skin pigmentation at and around the application site. Loss of pigmentation, in some cases, has been reported at other sites distant from the application site. Monitor for signs of skin depigmentation. Discontinue Methylphenidate Transdermal System if it occurs.
- Contact Sensitization: Use of Methylphenidate Transdermal System may lead to contact sensitization. Treatment should be discontinued if contact sensitization is suspected. Erythema is commonly seen with use of Methylphenidate Transdermal System and is not by itself an indication of sensitization. However, contact sensitization should be suspected if erythema is accompanied by evidence of a more intense local reaction (edema, papules, vesicles) that does not significantly improve within 48 hours or spreads beyond the transdermal system site.
- External Heat: Patients should be advised to avoid exposing the Methylphenidate Transdermal System application site to direct external heat sources. When heat is applied to Methylphenidate Transdermal System after application, both the rate and extent of absorption are significantly increased.
- Hematologic monitoring: Periodic CBC, differential, and platelet counts are advised during prolonged therapy.
- Acute Angle Closure Glaucoma: Methylphenidate Transdermal System -treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist.
- Increased Intraocular Pressure (IOP) and Glaucoma: Prescribe Methylphenidate Transdermal System to patients with open-angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor patients with a history of increased IOP or open angle glaucoma.
- Motor and Verbal Tics and Worsening of Tourette’s Syndrome: Before initiating Methylphenidate Transdermal System, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor patients for the emergence or worsening of tics or Tourette’s syndrome. Discontinue treatment if clinically appropriate.
Methylphenidate Transdermal System has a high potential for abuse and misuse. The use of Methylphenidate Transdermal System exposes individuals to the risks of abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Methylphenidate Transdermal System can be diverted for non-medical use into illicit channels or distribution
Before prescribing Methylphenidate Transdermal System, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their caregivers or families about these risks. Advise patients to store Methylphenidate Transdermal System in a safe place, preferably locked, and instruct patients to not give Methylphenidate Transdermal System to anyone else. Throughout Methylphenidate Transdermal System treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
Methylphenidate Transdermal System has special disposal instructions. Instruct patients to find a take back location to dispose of unused or expired Methylphenidate Transdermal System. If a take back program is unavailable, instruct them to:
- Remove Methylphenidate Transdermal System from its pouch, separate it from its liner, fold it in half with the adhesive sides touching each other, and immediately flush the used transdermal system down the toilet, and
- Place the pouch and liner in a container, close the container, and throw out the container in the trash (advise patients not to flush the pouch and liner down the toilet).
Sudden death has been reported in patients with structural cardiac abnormalities or other serious cardiac disease who were treated with CNS stimulants at the recommended ADHD dosage.
Avoid Methylphenidate Transdermal System use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmia, coronary artery disease, or other serious cardiac disease.
CNS stimulants may cause an increase in blood pressure (mean increase approximately 2 to 4 mmHg) and heart rate (mean increase approximately 3 to 6 bpm). Some patients may have larger increases.
Monitor all Methylphenidate Transdermal System -treated patients for hypertension and tachycardia.
CNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.
CNS stimulants may induce a manic or mixed episode in patients. Prior to initiating Methylphenidate Transdermal System treatment, screen patients for risk factors for developing a manic episode (e.g., comorbid or history of depressive symptoms or a family history of suicide, bipolar disorder, or depression).
CNS stimulants, at the recommended dosages, may cause psychotic or manic symptoms, (e.g., hallucinations, delusional thinking, or mania) in patients without a prior history of psychotic illness or mania. In a pooled analysis of multiple short-term, placebo-controlled studies of CNS stimulants, psychotic or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated patients compared with 0% of placebo-treated patients. If such symptoms occur, consider discontinuing Methylphenidate Transdermal System.
There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in absence of seizures, and, very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, the drug should be discontinued.
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate use in both adult and pediatric male patients. Although priapism was not reported with methylphenidate initiation, it developed after some time on the methylphenidate, often subsequent to an increase in dosage. Priapism also occurred during methylphenidate withdrawal (drug holidays or during discontinuation).
Methylphenidate Transdermal System -treated patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
Stimulant medications, including Methylphenidate Transdermal System, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Signs and symptoms are usually intermittent and mild; however, sequelae have included digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud’s phenomenon, were observed in post-marketing reports and at therapeutic dosages of CNS stimulants in all age groups throughout the course of treatment. Signs and symptoms generally improved after dosage reduction or discontinuation of the CNS stimulant.
Careful observation for digital changes is necessary during Methylphenidate Transdermal System treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for Methylphenidate Transdermal System -treated patients who develop signs or symptoms of peripheral vasculopathy.
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated children over 36 months (to the ages of 10 to 13 years), suggests that pediatric patients who received methylphenidate for 7 days per week throughout the year had a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this development period.
Closely monitor growth (weight and height) in Methylphenidate Transdermal System -treated pediatric patients. Pediatric patients not growing or gaining height or weight as expected may need to have their treatment interrupted.
Methylphenidate Transdermal System use may result in a persistent loss of skin pigmentation at and around the application site. Loss of pigmentation, in some cases, has been reported at other sites distant from the application site. Chemical leukoderma can mimic the appearance of vitiligo, particularly when the loss of skin pigmentation involves areas distant from the application site. Individuals with a history of vitiligo and/or a family history of vitiligo may be more at risk. Skin depigmentation may persist even after Methylphenidate Transdermal System use is discontinued. Monitor for signs of skin depigmentation, and advise patients to immediately inform their healthcare provider if changes in skin pigmentation occur. Discontinue use of the Methylphenidate Transdermal System in patients with chemical leukoderma.
In an open-label study of 305 subjects conducted to characterize dermal reactions in children with ADHD treated with Methylphenidate Transdermal System using a 9-hour wear time, one subject (0.3%) was confirmed by patch testing to be sensitized to methylphenidate (allergic contact dermatitis). This subject experienced erythema and edema at Methylphenidate Transdermal System application sites with concurrent urticarial lesions on the abdomen and legs resulting in treatment discontinuation. This subject was not transitioned to oral methylphenidate.
Use of Methylphenidate Transdermal System may lead to contact sensitization. Methylphenidate Transdermal System should be discontinued if contact sensitization is suspected. Erythema is commonly seen with use of Methylphenidate Transdermal System and is not by itself an indication of sensitization. However, contact sensitization should be suspected if erythema is accompanied by evidence of a more intense local reaction (edema, papules, vesicles) that does not significantly improve within 48 hours or spreads beyond the transdermal system site. Confirmation of a diagnosis of contact sensitization (allergic contact dermatitis) may require further diagnostic testing.
Patients sensitized from use of Methylphenidate Transdermal System, as evidenced by development of an allergic contact dermatitis, may develop systemic sensitization or other systemic reactions if methylphenidate-containing products are taken via other routes, e.g., orally. Manifestations of systemic sensitization may include a flare-up of previous dermatitis or of prior positive patch-test sites, or generalized skin eruptions in previously unaffected skin. Other systemic reactions may include headache, fever, malaise, arthralgia, diarrhea, or vomiting. No cases of systemic sensitization have been observed in clinical trials of Methylphenidate Transdermal System.
Patients who develop contact sensitization to Methylphenidate Transdermal System and require oral treatment with methylphenidate should be initiated on oral medication under close medical supervision. It is possible that some patients sensitized to methylphenidate by exposure to Methylphenidate Transdermal System may not be able to take methylphenidate in any form.
Patients should be advised to avoid exposing the Methylphenidate Transdermal System application site to direct external heat sources, such as hair dryers, heating pads, electric blankets, heated water beds, etc., while wearing the transdermal system. When heat is applied to Methylphenidate Transdermal System after application, both the rate and extent of absorption are significantly increased. The temperature-dependent increase in methylphenidate absorption can be greater than 2-fold
Periodic CBC, differential, and platelet counts are advised during prolonged therapy.
There have been reports of angle closure glaucoma associated with methylphenidate treatment.
Although the mechanism is not clear, Methylphenidate Transdermal System -treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist.
There have been reports of an elevation of intraocular pressure (IOP) associated with methylphenidate treatment
Prescribe Methylphenidate Transdermal System to patients with open-angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor Methylphenidate Transdermal System-treated patients with a history of abnormally increased IOP or open angle glaucoma.
CNS stimulants, including methylphenidate, have been associated with the onset or exacerbation of motor and verbal tics. Worsening of Tourette’s syndrome has also been reported
Before initiating Methylphenidate Transdermal System, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor Methylphenidate Transdermal System -treated patients for the emergence or worsening of tics or Tourette’s syndrome, and discontinue treatment if clinically appropriate.
- Abuse, Misuse, and Addiction [see]
WARNING: ABUSE, MISUSE, AND ADDICTIONMethylphenidate Transdermal System has a high potential for abuse and misuse, which can lead to the development of substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including Methylphenidate Transdermal System, can result in overdose and death[see Overdosage ], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.Before prescribing Methylphenidate Transdermal System, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout Methylphenidate Transdermal System treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction[see Warnings and Precautions and Drug Abuse and Dependence ].WARNING: ABUSE, MISUSE, AND ADDICTION
See full prescribing information for complete boxed warningMethylphenidate Transdermal System has high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including Methylphenidate Transdermal System, can result in overdose and death :- Before prescribing Methylphenidate Transdermal System, assess each patient’s risk for abuse,misuse, and addiction.
- Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug.
- Throughout treatment, reassess each patient’s risk and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
- Hypersensitivity to Methylphenidate [see Contraindications ()]
4.1 Hypersensitivity to MethylphenidateMethylphenidate Transdermal System is contraindicated in patients known to be hypersensitive to methylphenidate or other components of the product (polyester/ethylene vinyl acetate laminate film backing, acrylic adhesive, silicone adhesive, and fluoropolymer-coated polyester)
[see Description ]. - Monoamine Oxidase Inhibitors [see Contraindications () and Drug Interactions (
4.2 Monoamine Oxidase InhibitorsMethylphenidate Transdermal System is contraindicated during treatment with monoamine oxidase inhibitors, and within a minimum of 14 days following discontinuation of treatment with a monoamine oxidase inhibitor (hypertensive crises may result).
)]7.1 Monoamine Oxidase Inhibitors (MAOI)Concomitant use of MAOIs and CNS stimulants, including Methylphenidate Transdermal System, can cause hypertensive crisis. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure
[see Contraindications ]. Concomitant use of Methylphenidate Transdermal System with MAOIs or within 14 days after discontinuing MAOI treatment is contraindicated. - Risks to Patients with Serious Cardiac Disease [see Warnings and Precautions ()]
5.2 Risks to Patients with Serious Cardiac DiseaseSudden death has been reported in patients with structural cardiac abnormalities or other serious cardiac disease who were treated with CNS stimulants at the recommended ADHD dosage.
Avoid Methylphenidate Transdermal System use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmia, coronary artery disease, or other serious cardiac disease.
- Increased Blood Pressure and Heart Rate [see Warnings and Precautions ()]
5.3 Increased Blood Pressure and Heart RateCNS stimulants may cause an increase in blood pressure (mean increase approximately 2 to 4 mmHg) and heart rate (mean increase approximately 3 to 6 bpm). Some patients may have larger increases.
Monitor all Methylphenidate Transdermal System -treated patients for hypertension and tachycardia.
- Psychiatric Adverse Reactions [see Warnings and Precautions ()]
5.4 Psychiatric Adverse ReactionsExacerbation of Pre-Existing PsychosisCNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.
Induction of a Manic Episode in Patients with Bipolar DiseaseCNS stimulants may induce a manic or mixed episode in patients. Prior to initiating Methylphenidate Transdermal System treatment, screen patients for risk factors for developing a manic episode (e.g., comorbid or history of depressive symptoms or a family history of suicide, bipolar disorder, or depression).
New Psychotic or Manic SymptomsCNS stimulants, at the recommended dosages, may cause psychotic or manic symptoms, (e.g., hallucinations, delusional thinking, or mania) in patients without a prior history of psychotic illness or mania. In a pooled analysis of multiple short-term, placebo-controlled studies of CNS stimulants, psychotic or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated patients compared with 0% of placebo-treated patients. If such symptoms occur, consider discontinuing Methylphenidate Transdermal System.
- Seizures [see Warnings and Precautions ()]
5.5 SeizuresThere is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in absence of seizures, and, very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, the drug should be discontinued.
- Priapism [see Warnings and Precautions ()]
5.6 PriapismProlonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate use in both adult and pediatric male patients. Although priapism was not reported with methylphenidate initiation, it developed after some time on the methylphenidate, often subsequent to an increase in dosage. Priapism also occurred during methylphenidate withdrawal (drug holidays or during discontinuation).
Methylphenidate Transdermal System -treated patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
- Peripheral Vasculopathy [see Warnings and Precautions ()]
5.7 Peripheral Vasculopathy, including Raynaud’s phenomenonStimulant medications, including Methylphenidate Transdermal System, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Signs and symptoms are usually intermittent and mild; however, sequelae have included digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud’s phenomenon, were observed in post-marketing reports and at therapeutic dosages of CNS stimulants in all age groups throughout the course of treatment. Signs and symptoms generally improved after dosage reduction or discontinuation of the CNS stimulant.
Careful observation for digital changes is necessary during Methylphenidate Transdermal System treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for Methylphenidate Transdermal System -treated patients who develop signs or symptoms of peripheral vasculopathy.
- Long-Term Suppression of Growth in Pediatric Patients [see Warnings and Precautions ()]
5.8 Long-Term Suppression of Growth in Pediatric PatientsMethylphenidate Transdermal System is not approved for use and is not recommended in pediatric patients below 6 years of age[see Use in Specific Populations ].CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated children over 36 months (to the ages of 10 to 13 years), suggests that pediatric patients who received methylphenidate for 7 days per week throughout the year had a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this development period.
Closely monitor growth (weight and height) in Methylphenidate Transdermal System -treated pediatric patients. Pediatric patients not growing or gaining height or weight as expected may need to have their treatment interrupted.
- Chemical Leukoderma [see Warnings and Precautions ()]
5.9 Chemical LeukodermaMethylphenidate Transdermal System use may result in a persistent loss of skin pigmentation at and around the application site. Loss of pigmentation, in some cases, has been reported at other sites distant from the application site. Chemical leukoderma can mimic the appearance of vitiligo, particularly when the loss of skin pigmentation involves areas distant from the application site. Individuals with a history of vitiligo and/or a family history of vitiligo may be more at risk. Skin depigmentation may persist even after Methylphenidate Transdermal System use is discontinued. Monitor for signs of skin depigmentation, and advise patients to immediately inform their healthcare provider if changes in skin pigmentation occur. Discontinue use of the Methylphenidate Transdermal System in patients with chemical leukoderma.
- Contact Sensitization [see Warnings and Precautions ()]
5.10 Contact SensitizationIn an open-label study of 305 subjects conducted to characterize dermal reactions in children with ADHD treated with Methylphenidate Transdermal System using a 9-hour wear time, one subject (0.3%) was confirmed by patch testing to be sensitized to methylphenidate (allergic contact dermatitis). This subject experienced erythema and edema at Methylphenidate Transdermal System application sites with concurrent urticarial lesions on the abdomen and legs resulting in treatment discontinuation. This subject was not transitioned to oral methylphenidate.
Use of Methylphenidate Transdermal System may lead to contact sensitization. Methylphenidate Transdermal System should be discontinued if contact sensitization is suspected. Erythema is commonly seen with use of Methylphenidate Transdermal System and is not by itself an indication of sensitization. However, contact sensitization should be suspected if erythema is accompanied by evidence of a more intense local reaction (edema, papules, vesicles) that does not significantly improve within 48 hours or spreads beyond the transdermal system site. Confirmation of a diagnosis of contact sensitization (allergic contact dermatitis) may require further diagnostic testing.
Patients sensitized from use of Methylphenidate Transdermal System, as evidenced by development of an allergic contact dermatitis, may develop systemic sensitization or other systemic reactions if methylphenidate-containing products are taken via other routes, e.g., orally. Manifestations of systemic sensitization may include a flare-up of previous dermatitis or of prior positive patch-test sites, or generalized skin eruptions in previously unaffected skin. Other systemic reactions may include headache, fever, malaise, arthralgia, diarrhea, or vomiting. No cases of systemic sensitization have been observed in clinical trials of Methylphenidate Transdermal System.
Patients who develop contact sensitization to Methylphenidate Transdermal System and require oral treatment with methylphenidate should be initiated on oral medication under close medical supervision. It is possible that some patients sensitized to methylphenidate by exposure to Methylphenidate Transdermal System may not be able to take methylphenidate in any form.
- External Heat [see Warnings and Precautions ()]
5.11 Patients Using External HeatPatients should be advised to avoid exposing the Methylphenidate Transdermal System application site to direct external heat sources, such as hair dryers, heating pads, electric blankets, heated water beds, etc., while wearing the transdermal system. When heat is applied to Methylphenidate Transdermal System after application, both the rate and extent of absorption are significantly increased. The temperature-dependent increase in methylphenidate absorption can be greater than 2-fold
[see Clinical Pharmacology ]. This increased absorption can be clinically significant and can result in overdose of methylphenidate[see Overdosage ]. - Hematologic Monitoring [see Warnings and Precautions ()]
5.12 Hematologic MonitoringPeriodic CBC, differential, and platelet counts are advised during prolonged therapy.
- Acute Angle Closure Glaucoma [see Warnings and Precautions ()]
5.13 Acute Angle Closure GlaucomaThere have been reports of angle closure glaucoma associated with methylphenidate treatment.
Although the mechanism is not clear, Methylphenidate Transdermal System -treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist.
- Increased Intraocular Pressure and Glaucoma [see Warnings and Precautions ()]
5.14 Increased Intraocular Pressure and GlaucomaThere have been reports of an elevation of intraocular pressure (IOP) associated with methylphenidate treatment
[see Adverse Reactions ].Prescribe Methylphenidate Transdermal System to patients with open-angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor Methylphenidate Transdermal System-treated patients with a history of abnormally increased IOP or open angle glaucoma.
- Motor and Verbal Tics, and Worsening of Tourette’s Syndrome [see Warnings and Precautions ()]
5.15 Motor and Verbal Tics, and Worsening of Tourette’s SyndromeCNS stimulants, including methylphenidate, have been associated with the onset or exacerbation of motor and verbal tics. Worsening of Tourette’s syndrome has also been reported
[see Adverse Reactions ].Before initiating Methylphenidate Transdermal System, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor Methylphenidate Transdermal System -treated patients for the emergence or worsening of tics or Tourette’s syndrome, and discontinue treatment if clinically appropriate.
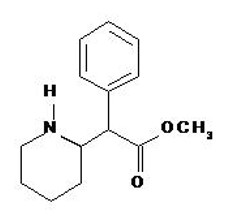
Methylphenidate Transdermal System is an adhesive-based matrix transdermal system containing methylphenidate that is applied to intact skin. The chemical name for methylphenidate is α-phenyl-2-piperidineacetic acid methyl ester. It is a white to off-white powder and is soluble in alcohol, ethyl acetate, and ether. Methylphenidate is practically insoluble in water and petrol ether. Its molecular weight is 233.31. Its empirical formula is C14H19NO2. The structural formula of methylphenidate is:
Methylphenidate Transdermal System contains methylphenidate in a multipolymeric adhesive. The methylphenidate is dispersed in acrylic adhesive that is dispersed in a silicone adhesive. The composition per unit area of all dosage strengths is identical, and the total dose delivered is dependent on the transdermal system size and wear time.
Methylphenidate Transdermal System consists of three layers, as seen in the figure below (cross-section of the transdermal system).
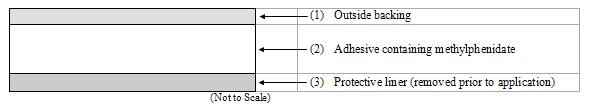
Proceeding from the outer surface toward the surface adhering to the skin, the layers are (1) a polyester/ethylene vinyl acetate laminate film backing, (2) a proprietary adhesive formulation incorporating Noven Pharmaceuticals, Inc.'s DOT Matrix™ transdermal technology consisting of an acrylic adhesive, a silicone adhesive, and methylphenidate, and (3) a fluoropolymer-coated polyester protective liner, which is attached to the adhesive surface and must be removed before the transdermal system can be used.
The active component of the transdermal system is methylphenidate. The remaining components are pharmacologically inactive.
Methylphenidate Transdermal System was demonstrated to be effective in the treatment of ADHD in two (2) randomized double-blind, placebo-controlled studies in children aged 6 to 12 years and one (1) randomized, double-blind, placebo-controlled study in adolescents aged 13 to 17 years who met Diagnostic and Statistical Manual (DSM-IV-TR®) criteria for ADHD. Methylphenidate Transdermal System wear time was 9 hours in all three (3) studies.
In Study 1, conducted in a classroom setting, symptoms of ADHD were evaluated by school teachers and observers using the Deportment Subscale from the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) rating scale which assesses behavior symptoms in the classroom setting. Methylphenidate Transdermal System was applied for 9 hours before removal. There was a 5-week open-label Methylphenidate Transdermal System dose optimization phase using dosages of 10, 15, 20, and 30 mg / 9 hours, followed by a 2-week randomized, double-blind, placebo-controlled crossover treatment phase using the optimal transdermal system dose for each patient or placebo. The mean differences between Methylphenidate Transdermal System and placebo in change from baseline in SKAMP Deportment Scores were statistically significant in favor of Methylphenidate Transdermal System beginning at 2 hours and remained statistically significant at all subsequent measured time points through 12 hours after application of the Methylphenidate Transdermal System.
In Study 2, conducted in the outpatient setting, Methylphenidate Transdermal System or placebo was blindly administered in a flexible-dose design using doses of 10, 15, 20, and 30 mg / 9 hours to achieve an optimal regimen over 5 weeks, followed by a 2-week maintenance period using the optimal transdermal system dose for each patient. Symptoms of ADHD were evaluated by the ADHD-Rating Scale (RS)-IV. Methylphenidate Transdermal System was statistically significantly superior to placebo as measured by the mean change from baseline for the ADHD-RS-IV total score. Although this study was not designed specifically to evaluate dose response, in general there did not appear to be any additional effectiveness accomplished by increasing the transdermal system dose from 20 mg / 9 hours to 30 mg / 9 hours.
In Study 3, conducted in the outpatient setting, Methylphenidate Transdermal System or placebo was blindly administered in a flexible-dose design using doses of 10, 15, 20, and 30 mg / 9 hours during a 5-week dose-optimization phase, followed by a 2-week maintenance period using the optimal transdermal system dose for each patient. Symptoms of ADHD were evaluated using the ADHD-Rating Scale (RS)-IV. Methylphenidate Transdermal System was statistically significantly superior to placebo as measured by the mean change from baseline in the ADHD-RS-IV total score.