Methyltestosterone
Methyltestosterone Prescribing Information
Androgens are indicated for replacement therapy in conditions associated with a deficiency or absence of endogenous testosterone:
- Primary hypogonadism (congenital or acquired) — testicular failure due to cryptorchidism, bilateral torsions, orchitis, vanishing testis syndrome; or orchidectomy.
- Hypogonadotropic hypogonadism (congenital or acquired) — gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency, or pituitary hypothalamic injury from tumors, trauma, or radiation. (Appropriate adrenal cortical and thyroid hormone replacement therapy are still necessary, however, and are actually of primary importance.) If the above conditions occur prior to puberty, androgen replacement therapy will be needed during the adolescent years for development of secondary sexual characteristics. Prolonged androgen treatment will be required to maintain sexual characteristics in these and other males who develop testosterone deficiency after puberty. Safety and efficacy of methyltestosterone in men with “age-related hypogonadism” (also referred to as “late-onset hypogonadism”) have not been established.
- Androgens may be used to stimulate puberty in carefully selected males with clearly delayed puberty. These patients usually have a familial pattern of delayed puberty that is not secondary to a pathological disorder; puberty is expected to occur spontaneously at a relatively late date. Brief treatment with conservative doses may occasionally be justified in these patients if they do not respond to psychological support. The potential adverse effect on bone maturation should be discussed with the patient and parents prior to androgen administration. An X-ray of the hand and wrist to determine bone age should be obtained every 6 months to assess the effect of treatment on the epiphyseal centers (see WARNINGS).
Prior to initiating methyltestosterone, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these serum testosterone concentrations are below the normal range.
Methyltestosterone capsules are administered orally. The suggested dosage for androgens varies depending on the age, sex, and diagnosis of the individual patient. Dosage is adjusted according to the patient’s response and the appearance of adverse reactions.
Replacement therapy in androgen-deficient males is 10 mg to 50 mg of methyltestosterone daily. Various dosage regimens have been used to induce pubertal changes in hypogonadal males, some experts have advocated lower dosages initially, gradually increasing the dose as puberty progresses with or without a decrease to maintenance levels. Other experts emphasize that higher dosages are needed to induce pubertal changes and lower dosages can be used for maintenance after puberty. The chronological and skeletal ages must be taken into consideration both in determining the initial dose and in adjusting the dose.
Doses used in delayed puberty generally are in the lower range of that given above, and for a limited duration, for example 4 to 6 months.
Women with metastatic breast carcinoma must be followed closely because androgen therapy occasionally appears to accelerate the disease. Thus, many experts prefer to use the shorter acting androgen preparations rather than those with prolonged activity for treating breast carcinoma, particularly during the early stages of androgen therapy. The dosage of methyltestosterone for androgen therapy in breast carcinoma in females is from 50 mg to 200 mg daily.
Androgens are contraindicated in men with carcinomas of the breast or with known or suspected carcinomas of the prostate, and in women who are or may become pregnant. When administered to pregnant women, androgens cause virilization of the external genitalia of the female fetus. This virilization includes clitoromegaly, abnormal vaginal development, and fusion of genital folds to form a scrotal-like structure. The degree of masculinization is related to the amount of drug given and the age of the fetus, and is most likely to occur in the female fetus when the drugs are given in the first trimester. If the patient becomes pregnant while taking these drugs, she should be apprised of the potential hazard to the fetus.
patients receiving oral anticoagulants. Patients receiving oral anticoagulant therapy require close monitoring, especially when androgens are started or stopped.
2. Oxyphenbutazone:
3. Insulin:
The androgens are steroids that develop and maintain primary and secondary male sex characteristics.
Androgens are derivatives of cyclopentanoper-hydrophenanthrene. Endogenous androgens are C-19 steroids with a side chain at C-17, and with two angular methyl groups. Testosterone is the primary endogenous androgen. In their active form, all drugs in the class have a 17-beta hydroxy group. 17-alpha alkylation (methyltestosterone) increases the pharmacologic activity per unit weight compared to testosterone when given orally.
Methyltestosterone, a synthetic derivative of testosterone, is an androgenic preparation given by the oral route in a capsule form. It has the following structural formula:
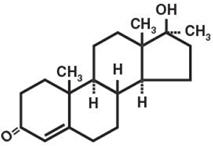
C20H30O2 M.W. 302.46
17-β-hydroxy-17-methylandrost-4-en-3-one
Methyltestosterone, USP occurs as white or creamy white crystals or powder, which is soluble in various organic solvents but is practically insoluble in water.
Each capsule, for oral administration, contains 10 mg of methyltestosterone, USP. In addition, each capsule contains the following inactive ingredients: corn starch, D&C Yellow #10, gelatin, FD&C Blue #1, FD&C Red #40, and magnesium stearate. Additionally, the capsule printing inks contain D&C Yellow #10, FD&C Blue #1, FD&C Blue #2, FD&C Red #40, iron oxide black, propylene glycol, and shellac.