Metoprolol Tartrate
Metoprolol Tartrate Prescribing Information
Metoprolol tartrate tablets, USP is supplied as:
The 25 mg tablets are White to off white round, biconvex tablets debossed with R 25 on one side and scored on the other side.
The 37.5 mg tablets are White to off white round, biconvex tablets debossed with R 375 on one side and scored on the other side.
The 50 mg tablets are White to off white round, biconvex tablets debossed with R 50 on one side and scored on the other side.
The 75 mg tablets are White to off white round, biconvex tablets debossed with R 75 on one side and scored on the other side.
The 100 mg tablets are White to off white round, biconvex tablets debossed with R 100 on one side and scored on the other side.
Metoprolol tartrate is contraindicated in severe bradycardia, second or third degree heart block, cardiogenic shock, systolic blood pressure <100, decompensated heart failure, sick sinus syndrome (unless a permanent pacemaker is in place), and in patients who are hypersensitive to any component of this product.
The following adverse reactions are described elsewhere in labeling:
- Worsening angina or myocardial infarction[see]
5 WARNINGS AND PRECAUTIONS- Abrupt cessation may exacerbate myocardial ischemia.
- Heart Failure: Worsening cardiac failure may occur.
- Bronchospastic Disease: Avoid beta-blockers.
- Pheochromocytoma: Initiate therapy with an alpha blocker.
- Major Surgery: Avoid initiation of high-dose extended-release metoprolol in patients undergoing non- cardiac surgery. Do not routinely withdraw chronic beta-blocker therapy prior to surgery.
- Diabetes: May mask symptoms of hypoglycemia.
- Thyrotoxicosis: Abrupt withdrawal in patients with thyrotoxicosis might precipitate a thyroid storm.
- Peripheral Vascular Disease: Can aggravate symptoms of arterial insufficiency.
5.1 Abrupt Cessation of TherapyFollowing abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered Metoprolol tartrate, particularly in patients with ischemic heart disease, gradually reduce the dosage over a period of 1 to 2 weeks and monitor the patient. If angina markedly worsens or acute coronary ischemia develops, promptly reinstate Metoprolol tartrate, and take measures appropriate for the management of unstable angina. Warn patients not to interrupt therapy without their physician’s advice. Because coronary artery disease is common and may be unrecognized, avoid abruptly discontinuing Metoprolol tartrate in patients treated only for hypertension.
5.2 Heart FailureWorsening cardiac failure may occur during up-titration of Metoprolol tartrate. If such symptoms occur, increase diuretics and restore clinical stability before advancing the dose of Metoprolol tartrate
[see Dosage and Administration (2)].It may be necessary to lower the dose of Metoprolol tartrate or temporarily discontinue it. Such episodes do not preclude subsequent successful titration of Metoprolol tartrate.5.3 Bronchospastic DiseasePatients with bronchospastic disease, should in general, not receive beta-blockers, including Metoprolol tartrate. Because of its relative beta1cardio-selectivity, however, Metoprolol tartrate may be used in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Because beta1-selectivity is not absolute, use the lowest possible dose of Metoprolol tartrate. Bronchodilators, including beta2-agonists, should be readily available or administered concomitantly
[see Dosage and Administration (2)].5.4 PheochromocytomaIf Metoprolol tartrate is used in the setting of pheochromocytoma, it should be given in combination with an alpha blocker, and only after the alpha blocker has been initiated. Administration of beta-blockers alone in the setting of pheochromocytoma has been associated with a paradoxical increase in blood pressure due to the attenuation of beta-mediated vasodilatation in skeletal muscle.
5.5 Major SurgeryAvoid initiation of a high-dose regimen of beta blocker therapy in patients undergoing non-cardiac surgery, since such use in patients with cardiovascular risk factors has been associated with bradycardia, hypotension, stroke and death.
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery, however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
5.6 HypoglycemiaBeta-blockers may prevent early warning signs of hypoglycemia, such as tachycardia, and increase the risk for severe or prolonged hypoglycemia at any time during treatment, especially in patients with diabetes mellitus or children and patients who are fasting (i.e., surgery, not eating regularly, or are vomiting). If severe hypoglycemia occurs, patients should be instructed to seek emergency treatment. Beta-blockers may mask some of the manifestations of hypoglycemia, particularly tachycardia.
5.7 ThyrotoxicosisBeta-adrenergic blockade may mask certain clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of beta-blockade may precipitate a thyroid storm.
5.8 Risk of Anaphylactic ReactionsWhile taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
5.9 Peripheral Vascular DiseaseBeta-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease.
- Worsening heart failure[see].
5 WARNINGS AND PRECAUTIONS- Abrupt cessation may exacerbate myocardial ischemia.
- Heart Failure: Worsening cardiac failure may occur.
- Bronchospastic Disease: Avoid beta-blockers.
- Pheochromocytoma: Initiate therapy with an alpha blocker.
- Major Surgery: Avoid initiation of high-dose extended-release metoprolol in patients undergoing non- cardiac surgery. Do not routinely withdraw chronic beta-blocker therapy prior to surgery.
- Diabetes: May mask symptoms of hypoglycemia.
- Thyrotoxicosis: Abrupt withdrawal in patients with thyrotoxicosis might precipitate a thyroid storm.
- Peripheral Vascular Disease: Can aggravate symptoms of arterial insufficiency.
5.1 Abrupt Cessation of TherapyFollowing abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered Metoprolol tartrate, particularly in patients with ischemic heart disease, gradually reduce the dosage over a period of 1 to 2 weeks and monitor the patient. If angina markedly worsens or acute coronary ischemia develops, promptly reinstate Metoprolol tartrate, and take measures appropriate for the management of unstable angina. Warn patients not to interrupt therapy without their physician’s advice. Because coronary artery disease is common and may be unrecognized, avoid abruptly discontinuing Metoprolol tartrate in patients treated only for hypertension.
5.2 Heart FailureWorsening cardiac failure may occur during up-titration of Metoprolol tartrate. If such symptoms occur, increase diuretics and restore clinical stability before advancing the dose of Metoprolol tartrate
[see Dosage and Administration (2)].It may be necessary to lower the dose of Metoprolol tartrate or temporarily discontinue it. Such episodes do not preclude subsequent successful titration of Metoprolol tartrate.5.3 Bronchospastic DiseasePatients with bronchospastic disease, should in general, not receive beta-blockers, including Metoprolol tartrate. Because of its relative beta1cardio-selectivity, however, Metoprolol tartrate may be used in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Because beta1-selectivity is not absolute, use the lowest possible dose of Metoprolol tartrate. Bronchodilators, including beta2-agonists, should be readily available or administered concomitantly
[see Dosage and Administration (2)].5.4 PheochromocytomaIf Metoprolol tartrate is used in the setting of pheochromocytoma, it should be given in combination with an alpha blocker, and only after the alpha blocker has been initiated. Administration of beta-blockers alone in the setting of pheochromocytoma has been associated with a paradoxical increase in blood pressure due to the attenuation of beta-mediated vasodilatation in skeletal muscle.
5.5 Major SurgeryAvoid initiation of a high-dose regimen of beta blocker therapy in patients undergoing non-cardiac surgery, since such use in patients with cardiovascular risk factors has been associated with bradycardia, hypotension, stroke and death.
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery, however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
5.6 HypoglycemiaBeta-blockers may prevent early warning signs of hypoglycemia, such as tachycardia, and increase the risk for severe or prolonged hypoglycemia at any time during treatment, especially in patients with diabetes mellitus or children and patients who are fasting (i.e., surgery, not eating regularly, or are vomiting). If severe hypoglycemia occurs, patients should be instructed to seek emergency treatment. Beta-blockers may mask some of the manifestations of hypoglycemia, particularly tachycardia.
5.7 ThyrotoxicosisBeta-adrenergic blockade may mask certain clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of beta-blockade may precipitate a thyroid storm.
5.8 Risk of Anaphylactic ReactionsWhile taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
5.9 Peripheral Vascular DiseaseBeta-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease.
- Worsening AV block[see].
4 CONTRAINDICATIONSMetoprolol tartrate is contraindicated in severe bradycardia, second or third degree heart block, cardiogenic shock, systolic blood pressure <100, decompensated heart failure, sick sinus syndrome (unless a permanent pacemaker is in place), and in patients who are hypersensitive to any component of this product.
- Known hypersensitivity to product components.
- Severe bradycardia: Greater than first degree heart block, or sick sinus syndrome without a pacemaker.
- Cardiogenic shock or decompensated heart failure.
Metoprolol tartrate tablets, USP contain metoprolol tartrate, a selective beta
1-adrenoreceptor blocking agent. Metoprolol tartrate is (±)-1- (Isopropylamino)-3-[p-(2-methoxyethyl) phenoxy]-2-propanol L-(+)-tartrate (2:1) salt, and its structural formula is
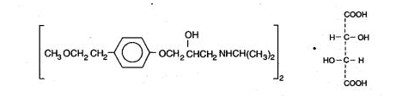
Metoprolol tartrate USP is a white, practically odorless, crystalline powder with a molecular weight of 684.82. It is very soluble in water; freely soluble in methylene chloride, in chloroform, and in alcohol; slightly soluble in acetone; and insoluble in ether.
Metoprolol tartrate tablets, USP is available as 25 mg, 37.5 mg, 50 mg, 75 mg, 100 mg tablets for oral administration containing 25 mg, 37.5 mg, 50 mg, 75 mg, 100 mg metoprolol tartrate.
Metoprolol Tartrate Tablets, USP are available containing 25 mg, 37.5 mg, 50 mg, 75 mg or 100 mg of metoprolol tartrate, USP.
The 25 mg tablets are White to off white round, biconvex tablets debossed with R 25 on one side and scored on the other side.
NDC 72888-208-01: Bottles of 100
NDC 72888-208-05: Bottles of 500
NDC 72888-208-00: Bottles of 1000
NDC 72888-208-02: 10000 in Pouch
The 37.5 mg tablets are White to off white round, biconvex tablets debossed with R 375 on one side and scored on the other side.
NDC 72888-209-01: Bottles of 100
NDC 72888-209-05: Bottles of 500
The 50 mg tablets are White to off white round, biconvex tablets debossed with R 50 on one side and scored on the other side.
NDC 72888-210-01: Bottles of 100
NDC 72888-210-05: Bottles of 500
NDC 72888-210-00: Bottles of 1000
NDC 72888-210-02: 5000 in Pouch
The 75 mg tablets are White to off white round, biconvex tablets debossed with R 75 on one side and scored on the other side.
NDC 72888-211-01: Bottles of 100
NDC 72888-211-05: Bottles of 500
The 100 mg tablets are White to off white round, biconvex tablets debossed with R 100 on one side and scored on the other side.
NDC 72888-212-01: Bottles of 100
NDC 72888-212-05: Bottles of 500
NDC 72888-212-00: Bottles of 1000
Store at 20° to 25°C (68° to 77°F). [
Protect from moisture.
Dispense in a tight, light-resistant container (USP) as defined in the USP using a child-resistant closure.