Metronidazole
(Metronidazole Topical)Metronidazole Prescribing Information
Metronidazole gel USP (topical), 0.75%, is indicated for topical application in the treatment of inflammatory papules and pustules of rosacea.
Apply and rub in a thin film of metronidazole gel USP (topical), 0.75%, twice daily, morning and evening, to entire affected areas after washing. Areas to be treated should be cleansed before application of metronidazole gel USP (topical), 0.75%. Patients may use cosmetics after application of metronidazole gel USP (topical), 0.75%.
Metronidazole gel USP (topical), 0.75%, is contraindicated in individuals with a history of hypersensitivity lo metronidazole, parabens, or other ingredients of the formulation.
The following adverse experiences have been reported with the topical use of metronidazole: burning, skin irritation, dryness, transient redness, metallic taste, tingling or numbness of extremities and nausea.
Oral metronidazole has been reported to potentiate the anticoagulant effect of coumarin and warfarin resulting in a prolongation of prothrombin time. The effect of topical metronidazole on prothrombin time is not known.
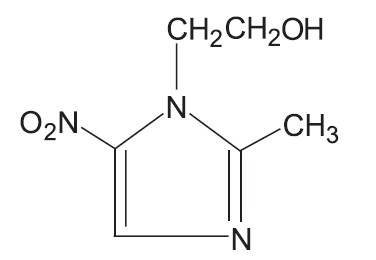
Metronidazole gel USP (topical), 0.75%, contains metronidazole, at a concentration of 7.5 mg per gram (0.75%) in a gel consisting of carbopol 980, edetate disodium, methylparaben, propylene glycol, propylparaben, purified water, and sodium hydroxide. Metronidazole is classified therapeutically as an antiprotozoal and anti-bacterial agent. Chemically, metronidazole is named 2-methyl-5-nitro-1H-imidazole-1- ethanol and has the following structure: