Milrinone Lactate - Milrinone Lactate injection, Solution
(Milrinone Lactate)Milrinone Lactate - Milrinone Lactate injection, Solution Prescribing Information
Milrinone lactate injection is indicated for the short-term intravenous treatment of patients with acute decompensated heart failure. Patients receiving milrinone should be observed closely with appropriate electrocardiographic equipment. The facility for immediate treatment of potential cardiac events, which may include life threatening ventricular arrhythmias, must be available. The majority of experience with intravenous milrinone has been in patients receiving digoxin and diuretics. There is no experience in controlled trials with infusions of milrinone for periods exceeding 48 hours.
Milrinone lactate injection should be administered with a loading dose followed by a continuous infusion (maintenance dose) according to the following guidelines:
50 mcg/kg: Administer slowly over 10 minutes
The table below shows the loading dose in milliliters (mL) of Milrinone lactate injection (1mg/mL) by patient body weight (kg).
Loading Dose (mL) Using 1 mg/mL Concentration | ||||||||||
Patient Body Weight (kg) | ||||||||||
kg | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 |
mL | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | 4.5 | 5 | 5.5 | 6 |
The loading dose may be given undiluted, but diluting to a rounded total volume of 10 or 20 mL (see Maintenance Dose for diluents) may simplify the visualization of the injection rate.
MAINTENANCE DOSE | |||
| Infusion Rate | Total Daily Dose (24 hours) |
|
Minimum | 0.375 mcg/kg/min | 0.59 mg/kg | Administer as a continuous intravenous infusion. |
Standard | 0.5 mcg/kg/min | 0.77 mg/kg | |
Maximum | 0.75 mcg/kg/min | 1.13 mg/kg | |
Milrinone lactate injection drawn from vials should be diluted prior to maintenance dose administration. The diluents that may be used are 0.45% Sodium Chloride Injection, USP; 0.9% Sodium Chloride Injection, USP; or 5% Dextrose Injection, USP. The table below shows the volume of diluent in milliliters (mL) that must be used to achieve 200 mcg/mL concentration for infusion, and the resultant total volumes.
Desired Infusion Concentration mcg/mL | Milrinone 1 mg/mL (mL) | Diluent (mL) | Total Volume (mL) |
200 | 10 | 40 | 50 |
200 | 20 | 80 | 100 |
The infusion rate should be adjusted according to hemodynamic and clinical response. Patients should be closely monitored. In controlled clinical studies, most patients showed an improvement in hemodynamic status as evidenced by increases in cardiac output and reductions in pulmonary capillary wedge pressure.
Note: See "
Data obtained from patients with severe renal impairment (creatinine clearance = 0 to 30 mL/min) but without congestive heart failure have demonstrated that the presence of renal impairment significantly increases the terminal elimination half-life of milrinone. Reductions in infusion rate may be necessary in patients with renal impairment. For patients with clinical evidence of renal impairment, the recommended infusion rate can be obtained from the following table:
Creatinine Clearance (mL/min/1.73 m2) | Infusion Rate (mcg/kg/min) |
5 | 0.2 |
10 | 0.23 |
20 | 0.28 |
30 | 0.33 |
40 | 0.38 |
50 | 0.43 |
Intravenous drug products should be inspected visually and should not be used if particulate matter or discoloration is present.
The maintenance dose in mL/hr by patient body weight (kg) may be determined by reference to the following table.
Milrinone Infusion Rate (mL) Using 200 mcg/mL Concentration | ||||||||||
Maintenance Dose (mcg/kg/min) | Patient Body Weight (kg) | |||||||||
30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 | |
0.375 | 3.4 | 4.5 | 5.6 | 6.8 | 7.9 | 9 | 10.1 | 11.3 | 12.4 | 13.5 |
0.4 | 3.6 | 4.8 | 6 | 7.2 | 8.4 | 9.6 | 10.8 | 12 | 13.2 | 14.4 |
0.5 | 4.5 | 6 | 7.5 | 9 | 10.5 | 12 | 13.5 | 15 | 16.5 | 18 |
0.6 | 5.4 | 7.2 | 9 | 10.8 | 12.6 | 14.4 | 16.2 | 18 | 19.8 | 21.6 |
0.7 | 6.3 | 8.4 | 10.5 | 12.6 | 14.7 | 16.8 | 18.9 | 21 | 23.1 | 25.2 |
0.75 | 6.8 | 9 | 11.3 | 13.5 | 15.8 | 18 | 20.3 | 22.5 | 24.8 | 27 |
When administering milrinone lactate by continuous infusion, it is advisable to use a calibrated electronic infusion device.
Milrinone is contraindicated in patients who are hypersensitive to it.
In patients receiving milrinone in Phase II and III clinical trials, ventricular arrhythmias were reported in 12.1%: Ventricular ectopic activity, 8.5%; nonsustained ventricular tachycardia, 2.8%; sustained ventricular tachycardia, 1% and ventricular fibrillation, 0.2% (2 patients experienced more than one type of arrhythmia). Holter recordings demonstrated that in some patients injection of milrinone increased ventricular ectopy, including nonsustained ventricular tachycardia. Life-threatening arrhythmias were infrequent and when present have been associated with certain underlying factors such as preexisting arrhythmias, metabolic abnormalities (e.g. hypokalemia), abnormal digoxin levels and catheter insertion. Milrinone was not shown to be arrhythmogenic in an electrophysiology study. Supraventricular arrhythmias were reported in 3.8% of the patients receiving milrinone. The incidence of both supraventricular and ventricular arrhythmias has not been related to the dose or plasma milrinone concentration.
Other cardiovascular adverse reactions include hypotension, 2.9% and angina/chest pain, 1.2%.
In the post-marketing experience, there have been rare cases of "torsades de pointes" reported.
Headaches, usually mild to moderate in severity, have been reported in 2.9% of patients receiving milrinone.
Other adverse reactions reported, but not definitely related to the administration of milrinone include hypokalemia, 0.6%; tremor, 0.4%; and thrombocytopenia, 0.4%.
In addition to adverse events reported from clinical trials, the following events have been reported from worldwide post-marketing experience with milrinone:
Isolated spontaneous reports of bronchospasm and anaphylactic shock.
Liver function test abnormalities and skin reactions such as rash.
Administration site conditions: Infusion site reaction.
No untoward clinical manifestations have been observed in limited experience with patients in whom milrinone was used concurrently with the following drugs: digitalis glycosides; lidocaine, quinidine; hydralazine, prazosin; isosorbide dinitrate, nitroglycerin; chlorthalidone, furosemide, hydrochlorothiazide, spironolactone; captopril; heparin, warfarin, diazepam, insulin; and potassium supplements.
There is an immediate chemical interaction which is evidenced by the formation of a precipitate when furosemide is injected into an intravenous line of an infusion of milrinone. Therefore, furosemide should not be administered in intravenous lines containing milrinone.
Milrinone Lactate Injection, is a member of a new class of bipyridine inotropic/vasodilator agents with phosphodiesterase inhibitor activity, distinct from digitalis glycosides or catecholamines. Milrinone lactate is designated chemically as 1,6-Dihydro-2-methyl-6-oxo[3,4'-bipyridine]-5-carbonitrile lactate and has the following structural formula:
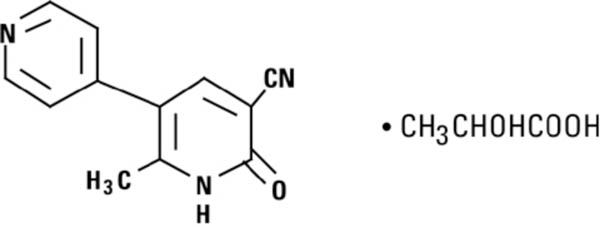
Milrinone is an off-white to tan crystalline compound with a molecular weight of 211.2 and a molecular formula of C12H9N3O. It is slightly soluble in methanol, and very slightly soluble in chloroform and in water. As the lactate salt, it is stable and colorless to pale yellow in solution. Milrinone lactate injection is available as a sterile aqueous solution of the lactate salt of milrinone for injection or infusion intravenously.