Moxifloxacin Prescribing Information
•
Fluoroquinolones, including moxifloxacin, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions can occur within hours to weeks after starting moxifloxacin. Patients of any age or without pre-existing risk factors have experienced these adverse reactions [see W
Discontinue moxifloxacin immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including moxifloxacin, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
•
Fluoroquinolones, including moxifloxacin, have been associated with an increased risk of tendinitis and tendon rupture in all ages [see
The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is increased in patients over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Other factors that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have also occurred in patients taking fluoroquinolones who do not have the above risk factors. Discontinue moxifloxacin immediately if the patient experiences pain, swelling, inflammation or rupture of a tendon.
Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug. Avoid fluoroquinolones, including moxifloxacin, in patients who have a history of tendon disorders or who have experienced tendinitis or tendon rupture [see
•
Fluoroquinolones, including moxifloxacin, have been associated with an increased risk of peripheral neuropathy. Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving fluoroquinolones including moxifloxacin. Symptoms may occur soon after initiation of moxifloxacin and may be irreversible in some patients [see
Discontinue moxifloxacin immediately if the patient experiences symptoms of peripheral neuropathy including pain, burning, tingling, numbness, and/or weakness or other alterations of sensation including light touch, pain, temperature, position sense, and vibratory sensation. Avoid fluoroquinolones, including moxifloxacin, in patients who have previously experienced peripheral neuropathy
•
Fluoroquinolones, including moxifloxacin, have been associated with an increased risk of psychiatric adverse reactions, including: toxic psychosis, hallucinations, or paranoia; depression or suicidal thoughts or acts; anxiety, agitation, or nervousness; confusion, delirium, disorientation, or disturbances in attention; insomnia or nightmares; memory impairment. These adverse reactions may occur following the first dose. If these reactions occur in patients receiving moxifloxacin, discontinue moxifloxacin immediately and institute appropriate measures [see Adverse Reactions ].
Fluoroquinolones, including moxifloxacin, have been associated with an increased risk of seizures (convulsions), increased intracranial pressure (including pseudotumor cerebri), dizziness, and tremors. As with all fluoroquinolones, use moxifloxacin with caution in patients with known or suspected CNS disorders (for example, severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold. These adverse reactions may occur following the first dose. If these reactions occur in patients receiving moxifloxacin, discontinue moxifloxacin immediately and institute appropriate measures [see Drug Interactions (7.4)Adverse Reactions , and Patient Counseling Information ].
Fluoroquinolones, including moxifloxacin, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions can occur within hours to weeks after starting moxifloxacin. Patients of any age or without pre-existing risk factors have experienced these adverse reactions [see W
Discontinue moxifloxacin immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including moxifloxacin, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
Fluoroquinolones, including moxifloxacin, have neuromuscular blocking activity and may exacerbate muscle weakness in patients with myasthenia gravis. Postmarketing serious adverse reactions, including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in patients with myasthenia gravis. Avoid moxifloxacin in patients with known history of myasthenia gravis.
•
Fluoroquinolones, including moxifloxacin, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions can occur within hours to weeks after starting moxifloxacin. Patients of any age or without pre-existing risk factors have experienced these adverse reactions [see W
Discontinue moxifloxacin immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including moxifloxacin, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
Prescribing moxifloxacin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
•
Moxifloxacin tablets are indicated in adult patients for the treatment of acute bacterial Sinusitis caused by susceptible isolates of
Because fluoroquinolones, including moxifloxacin tablets, have been associated with serious adverse reactions [see
Moxifloxacin tablets are indicated in adult patients for the treatment of Acute Bacterial Exacerbation of Chronic Bronchitis (ABECB) caused by susceptible isolates of
Because fluoroquinolones, including moxifloxacin tablets, have been associated with serious adverse reactions [see
Moxifloxacin tablets are contraindicated in persons with a history of hypersensitivity to moxifloxacin or any member of the quinolone class of antibacterials [see
Serious anaphylactic reactions, some following the first dose, have been reported in patients receiving fluoroquinolone therapy, including moxifloxacin. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, tingling, pharyngeal or facial edema, dyspnea, urticaria, and itching. Discontinue moxifloxacin at the first appearance of a skin rash or any other sign of hypersensitivity [see
The following serious and otherwise important adverse reactions are discussed in greater detail in the warnings and precautions section of the label:
• Disabling and Potentially Irreversible Serious Adverse Reactions Including Tendinitis and Tendon Rupture, Peripheral Neuropathy, and Central Nervous System Effects [see
Fluoroquinolones, including moxifloxacin, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions can occur within hours to weeks after starting moxifloxacin. Patients of any age or without pre-existing risk factors have experienced these adverse reactions [see W
Discontinue moxifloxacin immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including moxifloxacin, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
• Tendinitis and Tendon Rupture[see
Fluoroquinolones, including moxifloxacin, have been associated with an increased risk of tendinitis and tendon rupture in all ages [see
The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is increased in patients over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Other factors that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have also occurred in patients taking fluoroquinolones who do not have the above risk factors. Discontinue moxifloxacin immediately if the patient experiences pain, swelling, inflammation or rupture of a tendon.
Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug. Avoid fluoroquinolones, including moxifloxacin, in patients who have a history of tendon disorders or who have experienced tendinitis or tendon rupture [see
• Peripheral Neuropathy [see
Fluoroquinolones, including moxifloxacin, have been associated with an increased risk of peripheral neuropathy. Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving fluoroquinolones including moxifloxacin. Symptoms may occur soon after initiation of moxifloxacin and may be irreversible in some patients [see
Discontinue moxifloxacin immediately if the patient experiences symptoms of peripheral neuropathy including pain, burning, tingling, numbness, and/or weakness or other alterations of sensation including light touch, pain, temperature, position sense, and vibratory sensation. Avoid fluoroquinolones, including moxifloxacin, in patients who have previously experienced peripheral neuropathy
• Central Nervous System Effects [see
Fluoroquinolones, including moxifloxacin, have been associated with an increased risk of psychiatric adverse reactions, including: toxic psychosis, hallucinations, or paranoia; depression or suicidal thoughts or acts; anxiety, agitation, or nervousness; confusion, delirium, disorientation, or disturbances in attention; insomnia or nightmares; memory impairment. These adverse reactions may occur following the first dose. If these reactions occur in patients receiving moxifloxacin, discontinue moxifloxacin immediately and institute appropriate measures [see Adverse Reactions ].
Fluoroquinolones, including moxifloxacin, have been associated with an increased risk of seizures (convulsions), increased intracranial pressure (including pseudotumor cerebri), dizziness, and tremors. As with all fluoroquinolones, use moxifloxacin with caution in patients with known or suspected CNS disorders (for example, severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold. These adverse reactions may occur following the first dose. If these reactions occur in patients receiving moxifloxacin, discontinue moxifloxacin immediately and institute appropriate measures [see Drug Interactions (7.4)Adverse Reactions , and Patient Counseling Information ].
• Exacerbation of Myasthenia Gravis [see
Fluoroquinolones, including moxifloxacin, have neuromuscular blocking activity and may exacerbate muscle weakness in patients with myasthenia gravis. Postmarketing serious adverse reactions, including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in patients with myasthenia gravis. Avoid moxifloxacin in patients with known history of myasthenia gravis.
• QT Prolongation [see
Moxifloxacin has been shown to prolong the QT interval of the electrocardiogram in some patients. Following oral dosing with 400 mg of moxifloxacin the mean (± SD) change in QTc from the pre-dose value at the time of maximum drug concentration was 6 msec (± 26) (n = 787). Following a course of daily intravenous dosing (400 mg; 1 hour infusion each day) the mean change in QTc from the Day 1 pre-dose value was 10 msec (±22) on Day 1 (n=667) and 7 msec (± 24) on Day 3 (n = 667).
Avoid moxifloxacin in patients with the following risk factors due to the lack of clinical experience with the drug in these patient populations:
• Known prolongation of the QT interval
• Ventricular arrhythmias including torsade de pointes because QT prolongation may lead to an increased risk for these conditions
• Ongoing proarrhythmic conditions, such as clinically significant bradycardia and acute myocardial ischemia,
• Uncorrected hypokalemia or hypomagnesemia
• Class IA (for example, quinidine, procainamide) or Class III (for example, amiodarone, sotalol) antiarrhythmic agents
• Other drugs that prolong the QT interval such as cisapride, erythromycin, antipsychotics, and tricyclic antidepressants
Elderly patients using intravenous moxifloxacin may be more susceptible to drug-associated QT prolongation [see
In patients with mild, moderate, or severe liver cirrhosis, metabolic disturbances associated with hepatic insufficiency may lead to QT prolongation. Monitor ECG in patients with liver cirrhosis treated with moxifloxacin [see
The magnitude of QT prolongation may increase with increasing concentrations of the drug or increasing rates of infusion of the intravenous formulation. Therefore, the recommended dose or infusion rate should not be exceeded.
In premarketing clinical trials, the rate of cardiovascular adverse reactions was similar in 798 moxifloxacin and 702 comparator treated patients who received concomitant therapy with drugs known to prolong the QTc interval. No excess in cardiovascular morbidity or mortality attributable to QTc prolongation occurred with moxifloxacin treatment in over 15,500 patients in controlled clinical studies, including 759 patients who were hypokalemic at the start of treatment, and there was no increase in mortality in over 18,000 moxifloxacin tablet treated patients in a postmarketing observational study in which ECGs were not performed.
• Other Serious and Sometimes Fatal Adverse Reactions [see
Other serious and sometimes fatal adverse reactions, some due to hypersensitivity, and some due to uncertain etiology, have been reported in patients receiving therapy with fluoroquinolones, including moxifloxacin. These reactions may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following:
• Fever, rash, or severe dermatologic reactions (for example, toxic epidermal necrolysis, Stevens-Johnson syndrome)
• Vasculitis; arthralgia; myalgia; serum sickness
• Allergic pneumonitis
• Interstitial nephritis; acute renal insufficiency or failure
• Hepatitis; jaundice; acute hepatic necrosis or failure
• Anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities
Discontinue moxifloxacin immediately at the first appearance of a skin rash, jaundice, or any other sign of hypersensitivity and institute supportive measures.
• Hypersensitivity Reactions [see
Serious anaphylactic reactions, some following the first dose, have been reported in patients receiving fluoroquinolone therapy, including moxifloxacin. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, tingling, pharyngeal or facial edema, dyspnea, urticaria, and itching. Discontinue moxifloxacin at the first appearance of a skin rash or any other sign of hypersensitivity [see
• Risk of Aortic Aneurysm and Dissection [see
Epidemiologic studies report an increased rate of aortic aneurysm and dissection within two months following use of fluoroquinolones, particularly in elderly patients. The cause for the increased risk has not been identified. In patients with a known aortic aneurysm or patients who are at greater risk for aortic aneurysms, reserve moxifloxacin for use only when there are no alternative antibacterial treatments available.
•
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against
• Blood Glucose Disturbances [see
As with all fluoroquinolones, disturbances in blood glucose, including both hypoglycemia and hyperglycemia have been reported with moxifloxacin. In moxifloxacin-treated patients, dysglycemia occurred predominantly in elderly diabetic patients receiving concomitant treatment with an oral hypoglycemic agent (for example, sulfonylurea) or with insulin. Severe cases of hypoglycemia resulting in coma or death have been reported. In diabetic patients, careful monitoring of blood glucose is recommended. If a hypoglycemic reaction occurs, discontinue moxifloxacin and initiate appropriate therapy immediately [see Adverse Reactions , Drug Interactions and Patient Counseling Information ].
• Photosensitivity/Phototoxicity [see
Moderate to severe photosensitivity/phototoxicity reactions, the latter of which may manifest as exaggerated sunburn reactions (for example, burning, erythema, exudation, vesicles, blistering, edema) involving areas exposed to light (typically the face, “V” area of the neck, extensor surfaces of the forearms, dorsa of the hands), can be associated with the use of fluoroquinolones, including moxifloxacin, after sun or UV light exposure. Therefore, excessive exposure to these sources of light should be avoided. Moxifloxacin therapy should be discontinued if phototoxicity occurs [see
• Development of Drug Resistant Bacteria [see
Prescribing moxifloxacin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
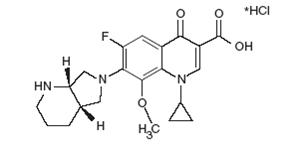
Moxifloxacin hydrochloride USP is a synthetic antibacterial agent for oral administration. Moxifloxacin, a fluoroquinolone, is available as the monohydrochloride salt of 1-cyclopropyl-7-[(S,S)-2,8-diazabicyclo[4.3.0]non-8-yl]-6-fluoro-8-methoxy-1,4-dihydro-4-oxo-3 quinoline carboxylic acid. It is a slightly yellow to yellow powder or crystals with a molecular weight of 437.9. Its molecular formula is C21H24FN3O4*HCl and its chemical structure is as follows
Moxifloxacin is a member of the flouroquinolone class of antibacterial agents [see
The bactericidal action of moxifloxacin results from inhibition of the topoisomerase II (DNA gyrase) and topoisomerase IV required for bacterial DNA replication, transcription, repair, and recombination.
The mechanism of action for fluoroquinolones, including moxifloxacin, is different from that of macrolides, beta-lactams, aminoglycosides, or tetracyclines; therefore, microorganisms resistant to these classes of drugs may be susceptible to moxifloxacin. Resistance to fluoroquinolones occurs primarily by a mutation in topoisomerase II (DNA gyrase) or topoisomerase IV genes, decreased outer membrane permeability or drug efflux. In vitro resistance to moxifloxacin develops slowly via multiple-step mutations. Resistance to moxifloxacin occurs in vitro at a general frequency of between 1.8 x 10-9to < 1 x 10-11for Gram-positive bacteria.
Cross-resistance has been observed between moxifloxacin and other fluoroquinolones against Gram-negative bacteria. Gram-positive bacteria resistant to other fluoroquinolones may, however, still be susceptible to moxifloxacin. There is no known cross-resistance between moxifloxacin and other classes of antimicrobials.
Antimicrobial Activity
Moxifloxacin has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections . [see
**MDRSP, Multi-drug resistant
The following in vitro data are available, but their clinical significance is unknown
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.