Mycophenolate Mofetil Prescribing Information
- Use during pregnancy is associated with increased risks of first trimester pregnancy loss and congenital malformations. Avoid if safer treatment options are available. Females of reproductive potential must be counseled regarding pregnancy prevention and planning[see., Use in Special Populations (8.1, 8.3)]
5.1 Embryofetal ToxicityUse of MMF during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, especially external ear and other facial abnormalities including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, kidney and nervous system. Females of reproductive potential must be made aware of these risks and must be counseled regarding pregnancy prevention and planning. Avoid use of MMF during pregnancy if safer treatment options are available
[see Use in Specific Populations (8.1, 8.3)]. - Increased risk of development of lymphoma and other malignancies, particularly of the skin[see.]
5.2 Lymphoma and Other MalignanciesPatients receiving immunosuppressants, including mycophenolate mofetil, are at increased risk of developing lymphomas and other malignancies, particularly of the skin
[see Adverse Reactions (6.1)].The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent. For patients with increased risk for skin cancer, exposure to sunlight and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen with a high protection factor.Post-transplant lymphoproliferative disorder (PTLD) developed in 0.4% to 1% of patients receiving mycophenolate mofetil (2 g or 3 g) with other immunosuppressive agents in controlled clinical trials of kidney, heart and liver transplant patients
[see Adverse Reactions (6.1)].The majority of PTLD cases appear to be related to Epstein Barr Virus (EBV) infection. The risk of PTLD appears greatest in those individuals who are EBV seronegative, a population which includes many young children. In pediatric patients, no other malignancies besides PTLD were observed in clinical trials[see Adverse Reactions (6.1)]. - Increased susceptibility to bacterial, viral, fungal and protozoal infections, including opportunistic infections and viral reactivation of hepatitis B and C, which may lead to hospitalizations and fatal outcomes[see.]
5.3 Serious InfectionsPatients receiving immunosuppressants, including mycophenolate mofetil, are at increased risk of developing bacterial, fungal, protozoal and new or reactivated viral infections, including opportunistic infections. The risk increases with the total immunosuppressive load. These infections may lead to serious outcomes, including hospitalizations and death
[see Adverse Reactions (6.1), (6.2)].Serious viral infections reported include:
- Polyomavirus-associated nephropathy (PVAN), especially due to BK virus infection
- JC virus-associated progressive multifocal leukoencephalopathy (PML), and
- Cytomegalovirus (CMV) infections: CMV seronegative transplant patients who receive an organ from a CMV seropositive donor are at highest risk of CMV viremia and CMV disease.
- Viral reactivation in patients infected with Hepatitis B and C
- COVID-19
Consider dose reduction or discontinuation of mycophenolate mofetil in patients who develop new infections or reactivate viral infections, weighing the risk that reduced immunosuppression represents to the functioning allograft.PVAN, especially due to BK virus infection, is associated with serious outcomes, including deteriorating renal function and renal graft loss
[see Adverse Reactions (6.2)].Patient monitoring may help detect patients at risk for PVAN.PML, which is sometimes fatal, commonly presents with hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia
[see Adverse Reactions (6.2)]. In immunosuppressed patients, physicians should consider PML in the differential diagnosis in patients reporting neurological symptoms.The risk of CMV viremia and CMV disease is highest among transplant recipients seronegative for CMV at time of transplant who receive a graft from a CMV seropositive donor. Therapeutic approaches to limiting CMV disease exist and should be routinely provided. Patient monitoring may help detect patients at risk for CMV disease.
Viral reactivation has been reported in patients infected with HBV or HCV. Monitoring infected patients for clinical and laboratory signs of active HBV or HCV infection is recommended.
Mycophenolate mofetil for injection is indicated for the prophylaxis of organ rejection, in adult and pediatric recipients 3 months of age and older of allogeneic kidney
The three
In all three
Mycophenolate mofetil, in combination with corticosteroids and cyclosporine, reduced (statistically significant at 0.05 level) the incidence of treatment failure within the first 6 months following transplantation (
USA Study (N=499 patients) | Mycophenolate Mofetil 2 g/day (n=167 patients) | Mycophenolate Mofetil 3 g/day (n=166 patients) | AZA 1 to 2 mg/kg/day (n=166 patients) |
All 3 groups received anti-thymocyte globulin induction, cyclosporine and corticosteroids | |||
All treatment failures | 31.1% | 31.3% | 47.6% |
Early termination without prior acute rejection | 9.6% | 12.7% | 6.0% |
Biopsy-proven rejection episode on treatment | 19.8% | 17.5% | 38.0% |
Europe/Canada/ Australia Study (N=503 patients) | Mycophenolate Mofetil 2 g/day (n=173 patients) | Mycophenolate Mofetil 3 g/day (n=164 patients) | AZA 100 to 150 mg/day (n=166 patients) |
No induction treatment administered; all 3 groups received cyclosporine and corticosteroids. | |||
All treatment failures | 38.2% | 34.8% | 50.0% |
Early termination without prior acute rejection | 13.9% | 15.2% | 10.2% |
Biopsy-proven rejection episode on treatment | 19.7% | 15.9% | 35.5% |
Europe Study (N=491 patients) | Mycophenolate Mofetil 2 g/day (n=165 patients) | Mycophenolate Mofetil 3 g/day (n=160 patients) | Placebo (n=166 patients) |
No induction treatment administered; all 3 groups received cyclosporine and corticosteroids. | |||
All treatment failures | 30.3% | 38.8% | 56.0% |
Early termination without prior acute rejection | 11.5% | 22.5% | 7.2% |
Biopsy-proven rejection episode on treatment | 17.0% | 13.8% | 46.4% |
*Does not include death and graft loss as reason for early termination. | |||
No advantage of mycophenolate mofetil at 12 months with respect to graft loss or patient death (combined) was established (
Study | Mycophenolate Mofetil 2 g/day | Mycophenolate Mofetil 3 g/day | Control (AZA or Placebo) |
USA | 8.5% | 11.5% | 12.2% |
Europe/Canada/Australia | 11.7% | 11.0% | 13.6% |
Europe | 8.5% | 10.0% | 11.5% |
One open-label, safety and pharmacokinetic study of mycophenolate mofetil oral suspension 600 mg/m2twice daily (up to 1 g twice daily) in combination with cyclosporine and corticosteroids was performed at centers in the United States (9), Europe (5) and Australia (1) in 100 pediatric patients (3 months to 18 years of age) for the prevention of renal allograft rejection. Mycophenolate mofetil was well tolerated in pediatric patients
A double-blind, randomized, comparative, parallel-group, multicenter study in primary
The analyses of the endpoints showed:
- Rejection: No difference was established between mycophenolate mofetil and AZA with respect to biopsy-proven rejection with hemodynamic compromise.
- Survival: Mycophenolate mofetil was shown to be at least as effective as AZA in preventing death or re-transplantation at 1 year (seeTable 15).
All Patients (ITT) | Treated Patients | |||
AZA N = 323 | Mycophenolate Mofetil N = 327 | AZA N = 289 | Mycophenolate Mofetil N = 289 | |
Biopsy-proven rejection with hemodynamic compromise at 6 monthsa | 121 (38%) | 120 (37%) | 100 (35%) | 92 (32%) |
Death or re-transplantation at 1 year | 49 (15.2%) | 42 (12.8%) | 33 (11.4%) | 18 (6.2%) |
aHemodynamic compromise occurred if any of the following criteria were met: pulmonary capillary wedge pressure > 20 mm or a 25% increase; cardiac index < 2.0 L/min/m2or a 25% decrease; ejection fraction< 30%; pulmonary artery oxygen saturation< 60% or a 25% decrease; presence of new S3gallop; fractional shortening was< 20% or a 25% decrease; inotropic support required to manage the clinical condition. | ||||
A double-blind, randomized, comparative, parallel-group, multicenter study in primary hepatic transplant recipients was performed at centers in the United States (16), in Canada (2), in Europe (4) and in Australia (1). The total number of patients enrolled was 565. Per protocol, patients received mycophenolate mofetil 1 g twice daily intravenously for up to 14 days followed by mycophenolate mofetil 1.5 g twice daily orally or AZA 1 to 2 mg/kg/day intravenously followed by AZA 1 to 2 mg/kg/day orally, in combination with cyclosporine (Neoral®) and corticosteroids as maintenance immunosuppressive therapy. The actual median oral dose of AZA on study was 1.5 mg/kg/day (range of 0.3 to 3.8 mg/kg/day) initially and 1.26 mg/kg/day (range of 0.3 to 3.8 mg/kg/day) at 12 months. The two primary endpoints were: (1) the proportion of patients who experienced, in the first 6 months post-transplantation, one or more episodes of biopsy-proven and treated rejection or death or re-transplantation, and (2) the proportion of patients who experienced graft loss (death or re-transplantation) during the first 12 months post-transplantation. Patients who prematurely discontinued treatment were followed for the occurrence of allograft rejection and for the occurrence of graft loss (death or re-transplantation) for 1 year.
In combination with corticosteroids and cyclosporine, mycophenolate mofetil demonstrated a lower rate of acute rejection at 6 months and a similar rate of death or re-transplantation at 1 year compared to AZA (
AZA N = 287 | Mycophenolate Mofetil N = 278 | |
Biopsy-proven, treated rejection at 6 months (includes death or re-transplantation) | 137 (47.7%) | 107 (38.5%) |
Death or re-transplantation at 1 year | 42 (14.6%) | 41 (14.7%) |
Mycophenolate Mofetil for Injection USP,
Allergic reactions to mycophenolate mofetil for injection have been observed; therefore, mycophenolate mofetil for injection is contraindicated in patients with a hypersensitivity to mycophenolate mofetil (MMF), mycophenolic acid (MPA) or any component of the drug product. Mycophenolate mofetil for injection is contraindicated in patients who are allergic to Polysorbate 80 (TWEEN).
The following adverse reactions are discussed in greater detail in other sections of the label:
- Embryofetal Toxicity [see]
5.1 Embryofetal ToxicityUse of MMF during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, especially external ear and other facial abnormalities including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, kidney and nervous system. Females of reproductive potential must be made aware of these risks and must be counseled regarding pregnancy prevention and planning. Avoid use of MMF during pregnancy if safer treatment options are available
[see Use in Specific Populations (8.1, 8.3)]. - Lymphomas and Other Malignancies [see]
5.2 Lymphoma and Other MalignanciesPatients receiving immunosuppressants, including mycophenolate mofetil, are at increased risk of developing lymphomas and other malignancies, particularly of the skin
[see Adverse Reactions (6.1)].The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent. For patients with increased risk for skin cancer, exposure to sunlight and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen with a high protection factor.Post-transplant lymphoproliferative disorder (PTLD) developed in 0.4% to 1% of patients receiving mycophenolate mofetil (2 g or 3 g) with other immunosuppressive agents in controlled clinical trials of kidney, heart and liver transplant patients
[see Adverse Reactions (6.1)].The majority of PTLD cases appear to be related to Epstein Barr Virus (EBV) infection. The risk of PTLD appears greatest in those individuals who are EBV seronegative, a population which includes many young children. In pediatric patients, no other malignancies besides PTLD were observed in clinical trials[see Adverse Reactions (6.1)]. - Serious Infections [see]
5.3 Serious InfectionsPatients receiving immunosuppressants, including mycophenolate mofetil, are at increased risk of developing bacterial, fungal, protozoal and new or reactivated viral infections, including opportunistic infections. The risk increases with the total immunosuppressive load. These infections may lead to serious outcomes, including hospitalizations and death
[see Adverse Reactions (6.1), (6.2)].Serious viral infections reported include:
- Polyomavirus-associated nephropathy (PVAN), especially due to BK virus infection
- JC virus-associated progressive multifocal leukoencephalopathy (PML), and
- Cytomegalovirus (CMV) infections: CMV seronegative transplant patients who receive an organ from a CMV seropositive donor are at highest risk of CMV viremia and CMV disease.
- Viral reactivation in patients infected with Hepatitis B and C
- COVID-19
Consider dose reduction or discontinuation of mycophenolate mofetil in patients who develop new infections or reactivate viral infections, weighing the risk that reduced immunosuppression represents to the functioning allograft.PVAN, especially due to BK virus infection, is associated with serious outcomes, including deteriorating renal function and renal graft loss
[see Adverse Reactions (6.2)].Patient monitoring may help detect patients at risk for PVAN.PML, which is sometimes fatal, commonly presents with hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia
[see Adverse Reactions (6.2)]. In immunosuppressed patients, physicians should consider PML in the differential diagnosis in patients reporting neurological symptoms.The risk of CMV viremia and CMV disease is highest among transplant recipients seronegative for CMV at time of transplant who receive a graft from a CMV seropositive donor. Therapeutic approaches to limiting CMV disease exist and should be routinely provided. Patient monitoring may help detect patients at risk for CMV disease.
Viral reactivation has been reported in patients infected with HBV or HCV. Monitoring infected patients for clinical and laboratory signs of active HBV or HCV infection is recommended.
- Blood Dyscrasias: Neutropenia, Pure Red Cell Aplasia [see]
5.4 Blood Dyscrasias:
Neutropenia and Pure Red Cell Aplasia (PRCA)Severe neutropenia [absolute neutrophil count (ANC) < 0.5 x 103/μL] developed in transplant patients receiving mycophenolate mofetil 3 g daily
[see Adverse Reactions (6.1)].Patients receiving mycophenolate mofetil should be monitored for neutropenia.Neutropenia has been observed most frequently in the period from 31 to 180 days post-transplant in patients treated for prevention of kidney, heart and liver rejection. The development of neutropenia may be related to mycophenolate mofetil itself, concomitant medications, viral infections, or a combination of these causes. If neutropenia develops (ANC < 1.3 x 103/μL), dosing with mycophenolate mofetil should be interrupted or the dose reduced, appropriate diagnostic tests performed, and the patient managed appropriately[see Dosage and Administration (2.5)].Patients receiving mycophenolate mofetil should be instructed to report immediately any evidence of infection, unexpected bruising, bleeding or any other manifestation of bone marrow depression.
Consider monitoring with complete blood counts weekly for the first month, twice monthly for the second and third months, and monthly for the remainder of the first year.
Cases of pure red cell aplasia (PRCA) have been reported in patients treated with mycophenolate mofetil in combination with other immunosuppressive agents. In some cases, PRCA was found to be reversible with dose reduction or cessation of mycophenolate mofetil therapy. In transplant patients, however, reduced immunosuppression may place the graft at risk.
- Gastrointestinal Complications [see]
5.5 Gastrointestinal ComplicationsGastrointestinal bleeding requiring hospitalization, ulceration and perforations were observed in clinical trials. Physicians should be aware of these serious adverse effects particularly when administering mycophenolate mofetil to patients with a gastrointestinal disease.
- Acute Inflammatory Syndrome Associated with Mycophenolate Products [see]
5.7 Acute
Inflammatory Syndrome Associated with Mycophenolate ProductsAcute inflammatory syndrome (AIS) has been reported with the use of MMF and mycophenolate products, and some cases have resulted in hospitalization. AIS is a paradoxical pro-inflammatory reaction characterized by fever, arthralgias, arthritis, muscle pain and elevated inflammatory markers including, C-reactive protein and erythrocyte sedimentation rate, without evidence of infection or underlying disease recurrence. Symptoms occur within weeks to months of initiation of treatment or a dose increase. After discontinuation, improvement of symptoms and inflammatory markers are usually observed within 24 to 48 hours.Monitor patients for symptoms and laboratory parameters of AIS when starting treatment with mycophenolate products or when increasing the dosage. Discontinue treatment and consider other treatment alternatives based on the risk and benefit for the patient.
Mycophenolate mofetil is an antimetabolite immunosuppressant. It is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor.
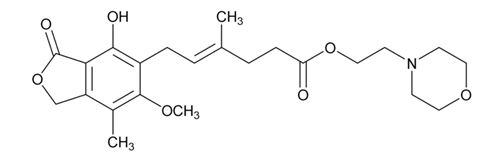
The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate. It has molecular formula of C23H31NO7, a molecular weight of 433.49 g/mol, and the following structural formula:
Mycophenolate mofetil, USP is a white to off-white crystalline powder. It is practically insoluble in water; the solubility increases in acidic medium (2.87 mg/mL at pH 1.2). It is freely soluble in acetone, soluble in methanol, and sparingly soluble in ethanol. The apparent partition coefficient in 1-octanol/water (pH 7.4) buffer solution is 2.42. The pKa values for MMF are 5.6 for the morpholino group and 8.5 for the phenolic group.
Mycophenolate mofetil hydrochloride has a solubility of 65.8 mg/mL in 5% Dextrose Injection USP (D5W). The pH of the reconstituted solution is 2.4 to 4.1.
Mycophenolate mofetil for injection, USP is the hydrochloride salt of mycophenolate mofetil, USP. The chemical name for the hydrochloride salt of mycophenolate mofetil is 2-morpholinoethyl (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate hydrochloride. It has molecular formula of C23H31NO7 HCl and a molecular weight of 469.96 g/mol.
Mycophenolate mofetil for injection, USP is available as a sterile, white to off-white lyophilized powder in single-dose vials containing mycophenolate mofetil hydrochloride for administration by intravenous infusion only. Each vial contains 500 mg of mycophenolate mofetil, USP equivalent to 542.1 mg of mycophenolate mofetil hydrochloride. The inactive ingredients are polysorbate 80, 25 mg; and citric acid anhydrous, 5 mg. Sodium hydroxide or hydrochloric acid may have been used in the manufacture of mycophenolate mofetil for injection, USP to adjust the pH. Reconstitution and dilution with 5% Dextrose Injection USP yields a slightly yellow solution of mycophenolate mofetil, 6 mg/mL
Instructions of Intravenous for Pharmacists
Mycophenolate mofetil (MMF) has demonstrated teratogenic effects in humans. Follow applicable special handling and disposal procedures1
Care should be taken to avoid direct contact with skin or mucous membranes of the powder contained in mycophenolate mofetil for injection because mycophenolate mofetil has demonstrated teratogenic effects in humans
Before proceeding with the preparation steps for mycophenolate mofetil for injection read the general preparation instructions
- Mycophenolate mofetil for injection does not contain an antibacterial preservative; therefore, reconstitution and dilution of the product must be performed under aseptic conditions.
- This product is sealed under vacuum and should retain a vacuum throughout its shelf life. If a lack of vacuum in the vial is noted while adding the diluent, the vial should not be used.
Mycophenolate mofetil for injection must be reconstituted and further diluted. A detailed description of the preparation is given below.
Preparation of the 1 g dose |
|
Preparation of the 1.5 g dose |
|
The administration of the infusion should be initiated within 4 hours of reconstitution and dilution of the drug product. Keep solutions at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). Discard unused portion of the reconstituted solutions.
Mycophenolate mofetil for injection should not be mixed or administered concurrently via the same infusion catheter with other intravenous drugs or infusion admixtures.