Mycophenolic Acid Prescribing Information
- Use during pregnancy is associated with increased risks of pregnancy loss and congenital malformations. Avoid if safer treatment options are available. Females of reproductive potential must be counseled regarding pregnancy prevention and planning[see, Use in Specific Populations (
5.1 Embryo-Fetal ToxicityUse of mycophenolic acid delayed‑release tablets during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, especially external ear and other facial abnormalities, including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, kidney, and nervous system. Females of reproductive potential must be aware of these risks and must be counseled regarding pregnancy prevention and planning. Avoid use of mycophenolic acid delayed‑release tablets during pregnancy if safer treatment options are available
[see Use in Specific Populations ].,8.1 PregnancyPregnancy Exposure RegistryThere is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to mycophenolate during pregnancy and those becoming pregnant within 6 weeks of discontinuing mycophenolic acid delayed‑release tablets treatment. To report a pregnancy or obtain information about the registry, visit www.mycophenolateREMS.com or call 1‑800‑617‑8191.
Risk SummaryFollowing oral or intravenous (IV) administration, MMF is metabolized to mycophenolic acid (MPA), the active ingredient in mycophenolic acid delayed-release tablets and the active form of the drug. Use of MMF during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of multiple congenital malformations in multiple organ systems
(see Human Data). Oral administration of mycophenolate to rats and rabbits during the period of organogenesis produced congenital malformations and pregnancy loss at doses less than the recommended clinical dose (0.05 and 1.1 times exposure at the recommended clinical doses in kidney transplant patients for rats and rabbits, respectively)(see Animal Data).Risks and benefits of mycophenolic acid delayed‑release tablets should be discussed with the patient. When appropriate, consider alternative immunosuppressants with less potential for embryo-fetal toxicity.
The estimated background risk of pregnancy loss and congenital malformations in organ transplant populations is not clear. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
DataHuman DataA spectrum of congenital malformations (including multiple malformations in individual newborns) has been reported in 23% to 27% of live births in MMF exposed pregnancies, based on published data from pregnancy registries. Malformations that have been documented include external ear, eye, and other facial abnormalities, including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, kidney, and nervous system. Based on published data from pregnancy registries, the risk of first trimester pregnancy loss has been reported at 45% to 49% following MMF exposure.
Animal DataIn animal reproductive toxicology studies, congenital malformations and pregnancy loss occurred when pregnant rats and rabbits received mycophenolate at dose multiples equivalent to and less than the recommended human dose. Oral administration of mycophenolate sodium to pregnant rats from Gestational Day 7 to Day 16 at a dose as low as 1 mg per kg resulted in malformations including anophthalmia, exencephaly, and umbilical hernia. The systemic exposure at this dose represents 0.05 times the clinical exposure at the human dose of 1,440 mg per day of mycophenolic acid delayed‑release tablets. Oral administration of mycophenolate to pregnant rabbits from Gestational Day 7 to Day 19 resulted in embryofetal lethality and malformations, including ectopia cordis, ectopic kidneys, diaphragmatic hernia, and umbilical hernia at doses equal to or greater than 80 mg per kg per day, in the absence of maternal toxicity. This corresponds to about 1.1 times the recommended clinical dose based on BSA.
)].8.3 Females and Males of Reproductive PotentialFemales of reproductive potential must be made aware of the increased risk of first trimester pregnancy loss and congenital malformations and must be counseled regarding pregnancy prevention and planning.
Pregnancy PlanningFor female patients taking mycophenolic acid delayed‑release tablets who are considering pregnancy, consider alternative immunosuppressants with less potential for embryo-fetal toxicity. Risks and benefits of mycophenolic acid delayed‑release tablets should be discussed with the patient.
Pregnancy TestingTo prevent unplanned exposure during pregnancy, females of reproductive potential should have a serum or urine pregnancy test with a sensitivity of at least 25 mIU/mL immediately before starting mycophenolic acid delayed‑release tablets. Another pregnancy test with the same sensitivity should be done 8 days to 10 days later. Repeat pregnancy tests should be performed during routine follow-up visits. Results of all pregnancy tests should be discussed with the patient. In the event of a positive pregnancy test, consider alternative immunosuppressants with less potential for embryo-fetal toxicity whenever possible.
ContraceptionFemale PatientsFemales of reproductive potential taking mycophenolic acid delayed‑release tablets must receive contraceptive counseling and use acceptable contraception (see Table 5 for Acceptable Contraception Methods). Patients must use acceptable birth control during entire mycophenolic acid delayed‑release tablets therapy, and for 6 weeks after stopping mycophenolic acid delayed‑release tablets, unless the patient chooses abstinence (she chooses to avoid heterosexual intercourse completely).
Patients should be aware that mycophenolic acid delayed‑release tablets reduce blood levels of the hormones in the oral contraceptive pill and could theoretically reduce its effectiveness
[see Patient Counseling Information (17), Drug Interactions (7.8)].Table 5: Acceptable Contraception Methods for Females of Reproductive Potential Pick from the following birth control options:Option 1Methods to Use AloneIntrauterine devices (IUDs)
Tubal sterilization
Patient’s partner had a vasectomyOR
Option 2Hormone Methods
choose 1Barrier Methods
choose 1Choose One Hormone MethodANDOne Barrier MethodEstrogen and Progesterone
Oral Contraceptive Pill
Transdermal patch
Vaginal ringProgesterone-only
Injection
ImplantANDDiaphragm with spermicide
Cervical cap with spermicide
Contraceptive sponge
Male condom
Female condomOR
Option 3Barrier Methods
choose 1Barrier Methods
choose 1Choose One Barrier Method
from each column(must choose
two methods)Diaphragm with spermicide
Cervical cap with spermicide
Contraceptive spongeANDMale condom
Female condomMale PatientsGenotoxic effects have been observed in animal studies at exposures exceeding the human therapeutic exposures by approximately 2.5 times. Thus, the risk of genotoxic effects on sperm cells cannot be excluded. Based on this potential risk, sexually active male patients and/or their female partners are recommended to use effective contraception during treatment of the male patient and for at least 90 days after cessation of treatment. Also, based on the potential risk of genotoxic effects, male patients should not donate sperm during treatment with mycophenolic acid delayed‑release tablets and for at least 90 days after cessation of treatment
[see Use in Specific Populations (8.1), Nonclinical Toxicology (13.1), Patient Counseling Information (17)]. - Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe mycophenolic acid delayed-release tablets. Patients receiving mycophenolic acid delayed-release tablets should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient[see].
5.2 Management of ImmunosuppressionOnly physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe mycophenolic acid delayed-release tablets. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physicians responsible for maintenance therapy should have complete information requisite for the follow-up of the patient
[see Boxed Warning]. - Increased risk of development of lymphoma and other malignancies, particularly of the skin, due to immunosuppression[see].
5.3 Lymphoma and Other MalignanciesPatients receiving immunosuppressants, including mycophenolic acid delayed‑release tablets, are at increased risk of developing lymphomas and other malignancies, particularly of the skin
[see Adverse Reactions (6)].The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent.As usual for patients with increased risk for skin cancer, exposure to sunlight and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen with a high protection factor.
Post-transplant lymphoproliferative disorder (PTLD) has been reported in immunosuppressed organ transplant recipients. The majority of PTLD events appear related to Epstein-Barr Virus (EBV) infection. The risk of PTLD appears greatest in those individuals who are EBV seronegative, a population which includes many young children.
- Increased susceptibility to bacterial, viral, fungal, and protozoal infections, including opportunistic infections[see Warnings and Precautions (,
5.4 Serious InfectionsPatients receiving immunosuppressants, including mycophenolic acid delayed‑release tablets, are at increased risk of developing bacterial, viral, fungal, and protozoal infections, and new or reactivated viral infections, including opportunistic infections
[see Warnings and Precautions (5.5)]. These infections may lead to serious, including fatal outcomes. Because of the danger of oversuppression of the immune system which can increase susceptibility to infection, combination immunosuppressant therapy should be used with caution.)].5.5 New or Reactivated Viral InfectionsPolyomavirus associated nephropathy (PVAN), JC virus-associated progressive multifocal leukoencephalopathy (PML), cytomegalovirus (CMV) infections, reactivation of hepatitis B (HBV) or hepatitis C (HCV), SARS- CoV-2 infection, have been reported in patients treated with immunosuppressants, including MPA derivatives mycophenolate sodium and MMF. Reduction in immunosuppression should be considered for patients who develop evidence of new or reactivated viral infections. Physicians should also consider the risk that reduced immunosuppression represents to the functioning allograft.
PVAN, especially due to BK virus infection, is associated with serious outcomes, including deteriorating renal function and renal graft loss. Patient monitoring may help detect patients at risk for PVAN.
PML, which is sometimes fatal, commonly presents with hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia. Risk factors for PML include treatment with immunosuppressant therapies and impairment of immune function. In immunosuppressed patients, physicians should consider PML in the differential diagnosis in patients reporting neurological symptoms and consultation with a neurologist should be considered as clinically indicated.
The risk of CMV viremia and CMV disease is highest among transplant recipients seronegative for CMV at time of transplant who receive a graft from a CMV seropositive donor. Therapeutic approaches to limiting CMV disease exist and should be routinely provided. Patient monitoring may help detect patients at risk for CMV disease
[see Adverse Reactions (6.1)].Viral reactivation has been reported in patients infected with HBV or HCV. Monitoring infected patients for clinical and laboratory signs of active HBV or HCV infection is recommended.
Mycophenolic acid is available as 360 mg and 180 mg delayed-release tablets.
Dosage Strength | 360 mg tablet | 180 mg tablet |
| Active ingredient | mycophenolic acid as mycophenolate sodium, USP | mycophenolic acid as mycophenolate sodium, USP |
| Appearance | Light-pink, film-coated, ovaloid, biconvex tablets | Light-green, film-coated, round, beveled edged, biconvex tablets |
| Imprint | “ aP36 ” on one side and plain on the other side. | “ aP18 ” on one side and plain on the other side. |
The following adverse reactions are discussed in greater detail in other sections of the label.
Embryo-Fetal Toxicity
[see,WARNING: EMBRYO-FETAL TOXICITY, MALIGNANCIES, and SERIOUS INFECTIONS- Use during pregnancy is associated with increased risks of pregnancy loss and congenital malformations. Avoid if safer treatment options are available. Females of reproductive potential must be counseled regarding pregnancy prevention and planning[see Warnings and Precautions (5.1), Use in Specific Populations ].
- Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe mycophenolic acid delayed-release tablets. Patients receiving mycophenolic acid delayed-release tablets should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient[see Warnings and Precautions (5.2)].
- Increased risk of development of lymphoma and other malignancies, particularly of the skin, due to immunosuppression[see Warnings and Precautions (5.3)].
- Increased susceptibility to bacterial, viral, fungal, and protozoal infections, including opportunistic infections[see Warnings and Precautions ].
WARNING: EMBRYO-FETAL TOXICITY, MALIGNANCIES, and SERIOUS INFECTIONSSee full prescribing information for complete boxed warningUse during pregnancy is associated with increased risks of pregnancy loss and congenital malformations. Avoid if safer treatment options are available. Females of reproductive potential must be counseled regarding pregnancy prevention and planning.
Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe mycophenolic acid delayed-release tablets.
Increased risk of development of lymphoma and other malignancies, particularly of the skin, due to immunosuppression.
Increased susceptibility to bacterial, viral, fungal, and protozoal infections, including opportunistic infections.
]5.1 Embryo-Fetal ToxicityUse of mycophenolic acid delayed‑release tablets during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, especially external ear and other facial abnormalities, including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, kidney, and nervous system. Females of reproductive potential must be aware of these risks and must be counseled regarding pregnancy prevention and planning. Avoid use of mycophenolic acid delayed‑release tablets during pregnancy if safer treatment options are available
[see Use in Specific Populations ].Lymphomas and Other Malignancies
[see,WARNING: EMBRYO-FETAL TOXICITY, MALIGNANCIES, and SERIOUS INFECTIONS- Use during pregnancy is associated with increased risks of pregnancy loss and congenital malformations. Avoid if safer treatment options are available. Females of reproductive potential must be counseled regarding pregnancy prevention and planning[see Warnings and Precautions (5.1), Use in Specific Populations ].
- Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe mycophenolic acid delayed-release tablets. Patients receiving mycophenolic acid delayed-release tablets should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient[see Warnings and Precautions (5.2)].
- Increased risk of development of lymphoma and other malignancies, particularly of the skin, due to immunosuppression[see Warnings and Precautions (5.3)].
- Increased susceptibility to bacterial, viral, fungal, and protozoal infections, including opportunistic infections[see Warnings and Precautions ].
WARNING: EMBRYO-FETAL TOXICITY, MALIGNANCIES, and SERIOUS INFECTIONSSee full prescribing information for complete boxed warningUse during pregnancy is associated with increased risks of pregnancy loss and congenital malformations. Avoid if safer treatment options are available. Females of reproductive potential must be counseled regarding pregnancy prevention and planning.
Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe mycophenolic acid delayed-release tablets.
Increased risk of development of lymphoma and other malignancies, particularly of the skin, due to immunosuppression.
Increased susceptibility to bacterial, viral, fungal, and protozoal infections, including opportunistic infections.
]5.3 Lymphoma and Other MalignanciesPatients receiving immunosuppressants, including mycophenolic acid delayed‑release tablets, are at increased risk of developing lymphomas and other malignancies, particularly of the skin
[see Adverse Reactions (6)].The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent.As usual for patients with increased risk for skin cancer, exposure to sunlight and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen with a high protection factor.
Post-transplant lymphoproliferative disorder (PTLD) has been reported in immunosuppressed organ transplant recipients. The majority of PTLD events appear related to Epstein-Barr Virus (EBV) infection. The risk of PTLD appears greatest in those individuals who are EBV seronegative, a population which includes many young children.
Serious Infections
[see,WARNING: EMBRYO-FETAL TOXICITY, MALIGNANCIES, and SERIOUS INFECTIONS- Use during pregnancy is associated with increased risks of pregnancy loss and congenital malformations. Avoid if safer treatment options are available. Females of reproductive potential must be counseled regarding pregnancy prevention and planning[see Warnings and Precautions (5.1), Use in Specific Populations ].
- Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe mycophenolic acid delayed-release tablets. Patients receiving mycophenolic acid delayed-release tablets should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient[see Warnings and Precautions (5.2)].
- Increased risk of development of lymphoma and other malignancies, particularly of the skin, due to immunosuppression[see Warnings and Precautions (5.3)].
- Increased susceptibility to bacterial, viral, fungal, and protozoal infections, including opportunistic infections[see Warnings and Precautions ].
WARNING: EMBRYO-FETAL TOXICITY, MALIGNANCIES, and SERIOUS INFECTIONSSee full prescribing information for complete boxed warningUse during pregnancy is associated with increased risks of pregnancy loss and congenital malformations. Avoid if safer treatment options are available. Females of reproductive potential must be counseled regarding pregnancy prevention and planning.
Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe mycophenolic acid delayed-release tablets.
Increased risk of development of lymphoma and other malignancies, particularly of the skin, due to immunosuppression.
Increased susceptibility to bacterial, viral, fungal, and protozoal infections, including opportunistic infections.
]5.4 Serious InfectionsPatients receiving immunosuppressants, including mycophenolic acid delayed‑release tablets, are at increased risk of developing bacterial, viral, fungal, and protozoal infections, and new or reactivated viral infections, including opportunistic infections
[see Warnings and Precautions (5.5)]. These infections may lead to serious, including fatal outcomes. Because of the danger of oversuppression of the immune system which can increase susceptibility to infection, combination immunosuppressant therapy should be used with caution.New or Reactivated Viral Infections
[see]5.5 New or Reactivated Viral InfectionsPolyomavirus associated nephropathy (PVAN), JC virus-associated progressive multifocal leukoencephalopathy (PML), cytomegalovirus (CMV) infections, reactivation of hepatitis B (HBV) or hepatitis C (HCV), SARS- CoV-2 infection, have been reported in patients treated with immunosuppressants, including MPA derivatives mycophenolate sodium and MMF. Reduction in immunosuppression should be considered for patients who develop evidence of new or reactivated viral infections. Physicians should also consider the risk that reduced immunosuppression represents to the functioning allograft.
PVAN, especially due to BK virus infection, is associated with serious outcomes, including deteriorating renal function and renal graft loss. Patient monitoring may help detect patients at risk for PVAN.
PML, which is sometimes fatal, commonly presents with hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia. Risk factors for PML include treatment with immunosuppressant therapies and impairment of immune function. In immunosuppressed patients, physicians should consider PML in the differential diagnosis in patients reporting neurological symptoms and consultation with a neurologist should be considered as clinically indicated.
The risk of CMV viremia and CMV disease is highest among transplant recipients seronegative for CMV at time of transplant who receive a graft from a CMV seropositive donor. Therapeutic approaches to limiting CMV disease exist and should be routinely provided. Patient monitoring may help detect patients at risk for CMV disease
[see Adverse Reactions (6.1)].Viral reactivation has been reported in patients infected with HBV or HCV. Monitoring infected patients for clinical and laboratory signs of active HBV or HCV infection is recommended.
Blood Dyscrasias, Including Pure Red Cell Aplasia
[see]5.6 Blood Dyscrasias, Including Pure Red Cell AplasiaCases of pure red cell aplasia (PRCA) have been reported in patients treated with MPA derivatives in combination with other immunosuppressive agents. The mechanism for MPA derivatives induced PRCA is unknown; the relative contribution of other immunosuppressants and their combinations in an immunosuppressive regimen is also unknown. In some cases, PRCA was found to be reversible with dose reduction or cessation of therapy with MPA derivatives. In transplant patients, however, reduced immunosuppression may place the graft at risk. Changes to mycophenolic acid delayed‑release tablets therapy should only be undertaken under appropriate supervision in transplant recipients in order to minimize the risk of graft rejection.
Patients receiving mycophenolic acid delayed‑release tablets should be monitored for blood dyscrasias (e.g., neutropenia or anemia). The development of neutropenia may be related to mycophenolic acid delayed‑release tablets itself, concomitant medications, viral infections, or some combination of these reactions. Complete blood count should be performed weekly during the first month, twice monthly for the second and the third month of treatment, then monthly through the first year. If blood dyscrasias occur [neutropenia develops (ANC < 1.3 × 103/mcL) or anemia], dosing with mycophenolic acid delayed‑release tablets should be interrupted or the dose reduced, appropriate tests performed, and the patient managed accordingly.
Serious GI Tract Complications
[see]5.7 Serious GI Tract ComplicationsGastrointestinal bleeding (requiring hospitalization), intestinal perforations, gastric ulcers, and duodenal ulcers have been reported in patients treated with mycophenolic acid delayed‑release tablets. Mycophenolic acid delayed‑release tablets should be administered with caution in patients with active serious digestive system disease.
Acute Inflammatory Syndrome Associated with Mycophenolate Products
[see]5.8 Acute Inflammatory Syndrome Associated with Mycophenolate ProductsAcute inflammatory syndrome (AIS) has been reported with the use of mycophenolate products, and some cases have resulted in hospitalization. AIS is a paradoxical pro-inflammatory reaction characterized by fever, arthralgias, arthritis, muscle pain and elevated inflammatory markers including, C-reactive protein and erythrocyte sedimentation rate, without evidence of infection or underlying disease recurrence. Symptoms occur within weeks to months of initiation of treatment or a dose increase. After discontinuation, improvement of symptoms and inflammatory markers are usually observed within 24 hours to 48 hours.
Monitor patients for symptoms and laboratory parameters of AIS when starting treatment with mycophenolate products or when increasing the dosage. Discontinue treatment and consider other treatment alternatives based on the risk and benefit for the patient.
Rare Hereditary Deficiencies
[see]5.10 Rare Hereditary DeficienciesMycophenolic acid is an inosine monophosphate dehydrogenase inhibitor (IMPDH inhibitor). Mycophenolic acid delayed‑release tablets should be avoided in patients with rare hereditary deficiency of hypoxanthine‑guanine phosphoribosyl‑transferase (HGPRT), such as Lesch‑Nyhan and Kelley‑Seegmiller syndromes because it may cause an exacerbation of disease symptoms characterized by the overproduction and accumulation of uric acid leading to symptoms associated with gout, such as acute arthritis, tophi, nephrolithiasis or urolithiasis, and renal disease, including renal failure.
Mycophenolic acid delayed-release tablets, USP are an enteric formulation of mycophenolate sodium, USP that delivers the active moiety mycophenolic acid (MPA). Mycophenolic acid is an immunosuppressive agent. As the sodium salt, MPA is chemically designated as (E)-6-(4‑hydroxy‑6-methoxy-7-methyl-3- oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoic acid sodium salt.
Its molecular formula is C17H19O6Na. The molecular weight is 342.32 g/mol and the structural formula is:
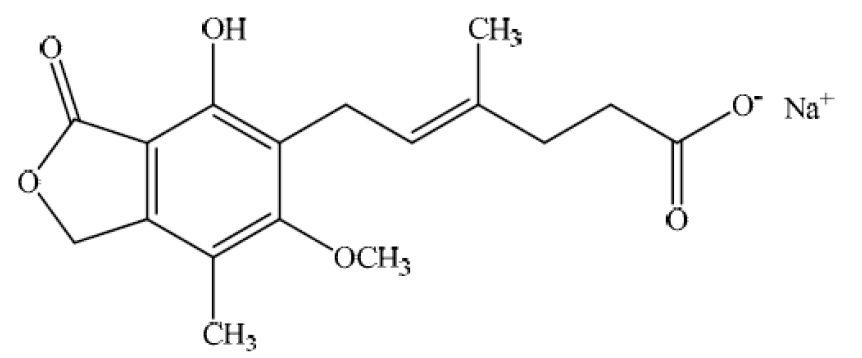
Mycophenolic acid, as the sodium salt, is a white to off-white, crystalline powder and is slightly soluble in water and practically insoluble in 0.1N hydrochloric acid.
Mycophenolic acid is available for oral use as delayed-release tablets containing either 180 mg or 360 mg of mycophenolic acid.
Inactive ingredients include anhydrous lactose, colloidal silicon dioxide, croscarmellose sodium, crospovidone, magnesium stearate, povidone (K-30), and pregelatinized starch. The enteric coating of the tablet consists of ferric oxide yellow, hypromellose phthalate, titanium dioxide, and FD&C blue no. 2 (180 mg) or ferric oxide red (360 mg). The imprinting ink consist of ammonium hydroxide, ferrosoferric oxide, propylene glycol, and shellac glaze.
Mycophenolic acid delayed-release tablets, USP are available in the strengths and packages listed below:
360 mg tablet: Light-pink, film-coated, ovaloid, biconvex tablets, imprinted with “
Bottles of 120............................................................................................ NDC 70377-127-11
180 mg tablet: Light-green, film-coated, round, beveled edged, biconvex tablets, imprinted with “
Bottles of 120............................................................................................ NDC 70377-126-11
Keep out of reach and sight of children. Mycophenolic acid delayed-release tablets should not be crushed or cut in order to maintain the integrity of the enteric coating
Mycophenolic acid delayed-release tablets should be taken on an empty stomach, 1 hour before or 2 hours after food intake
Mycophenolic acid delayed-release tablets should not be crushed, chewed, or cut prior to ingesting. The tablets should be swallowed whole in order to maintain the integrity of the enteric coating.
Pediatric patients with a BSA of 1.19 m2to 1.58 m2may be dosed either with three mycophenolic acid delayed‑release 180 mg tablets, or one 180 mg tablet plus one 360 mg tablet twice daily (1,080 mg daily dose). Patients with a BSA of > 1.58 m2may be dosed either with four mycophenolic acid delayed‑release 180 mg tablets, or two mycophenolic acid delayed‑release 360 mg tablets twice daily (1,440 mg daily dose). Pediatric doses for patients with BSA < 1.19 m2cannot be accurately administered using currently available formulations of mycophenolic acid delayed-release tablets.
Teratogenic effects have been observed with mycophenolate sodium
Use of mycophenolic acid delayed‑release tablets during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, especially external ear and other facial abnormalities, including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, kidney, and nervous system. Females of reproductive potential must be aware of these risks and must be counseled regarding pregnancy prevention and planning. Avoid use of mycophenolic acid delayed‑release tablets during pregnancy if safer treatment options are available