Myoview
(Tetrofosmin)Myoview Prescribing Information
MYOVIEW is a kit for the preparation of technetium Tc99m tetrofosmin for injection. Technetium Tc99m tetrofosmin injection is a radioactive diagnostic agent indicated for the following:
- Myocardial perfusion imaging under rest and/or exercise or pharmacologic stress conditions to delineate regions of reversible myocardial ischemia or infarcted myocardium in patients with known or suspected coronary artery disease ()
1.1 Myocardial Perfusion ImagingMyocardial perfusion imaging under rest and/or exercise or pharmacologic stress conditions to delineate regions of reversible myocardial ischemia or infarcted myocardium in patients with known or suspected coronary artery disease.
- Assessment of left ventricular function (left ventricular ejection fraction and wall motion) in patients with known or suspected heart disease ()
1.2 Ventricular Function ImagingMYOVIEW is indicated for assessment of left ventricular function (left ventricular ejection fraction and wall motion) in patients with known or suspected heart disease.
- Use appropriate radiation safety measures and aseptic technique during preparation and handling (,
2.1 Radiation Safety – Drug HandlingTechnetium Tc99m tetrofosmin is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure during administration
[see Warnings and Precautions (5.2)]. Use waterproof gloves and effective shielding, including syringe shields, when preparing and administering technetium Tc99m tetrofosmin injection.)2.3 Administration Instructions- Use aseptic technique for all drug preparation and handling.
- Measure the dose in a suitable radioactivity calibration system immediately prior to intravenous administration.
- Visually inspect the drug for particulate matter and discoloration prior to administration. Do not use or administer the drug if there is evidence of particulate matter or discoloration.
- Instruct patients to remain hydrated and void frequently following administration to decrease radiation exposure[see Warnings and Precautions (5.2)].
- The recommended dose range for MYOVIEW for rest or stress imaging is 185 to 1,221 megabecquerels (MBq) [5 to 33 millicuries (mCi)] by intravenous administration ()
2.2 Recommended Dosage- The recommended dose range for MYOVIEW is 185 to 1,221 megabecquerels (MBq) (5 to 33 millicuries (mCi) by intravenous administration for rest and stress imaging.
- When rest and stress intravenous injections are administered on the same day, the first dose should be 185 to 444 MBq (5 to 12 mCi) and followed by the second dose of 555 to 1,221 MBq (15 to 33 mCi) given approximately 1 to 4 hours later.
- The recommended dose range for MYOVIEW is 185 to 1,221 MBq (5 to 33 millicuries (mCi) by intravenous administration as an intravenous injection for ventricular function assessment.
- When rest and stress injections are administered on the same day, the first dose should be 185 to 444 MBq (5 to 12 mCi) followed by the second dose of 555 to 1,221 MBq (15 to 33 mCi) given approximately 1 to 4 hours later ()
2.2 Recommended Dosage- The recommended dose range for MYOVIEW is 185 to 1,221 megabecquerels (MBq) (5 to 33 millicuries (mCi) by intravenous administration for rest and stress imaging.
- When rest and stress intravenous injections are administered on the same day, the first dose should be 185 to 444 MBq (5 to 12 mCi) and followed by the second dose of 555 to 1,221 MBq (15 to 33 mCi) given approximately 1 to 4 hours later.
- The recommended dose range for MYOVIEW is 185 to 1,221 MBq (5 to 33 millicuries (mCi) by intravenous administration as an intravenous injection for ventricular function assessment.
- The recommended dose range for MYOVIEW for ventricular function assessment is 185 to 1,221 MBq (5 to 33 mCi) as an intravenous injection ()
2.2 Recommended Dosage- The recommended dose range for MYOVIEW is 185 to 1,221 megabecquerels (MBq) (5 to 33 millicuries (mCi) by intravenous administration for rest and stress imaging.
- When rest and stress intravenous injections are administered on the same day, the first dose should be 185 to 444 MBq (5 to 12 mCi) and followed by the second dose of 555 to 1,221 MBq (15 to 33 mCi) given approximately 1 to 4 hours later.
- The recommended dose range for MYOVIEW is 185 to 1,221 MBq (5 to 33 millicuries (mCi) by intravenous administration as an intravenous injection for ventricular function assessment.
- See Full Prescribing Information for instructions for preparation and determination of radiochemical purity (,
2.4 Instructions for PreparationThe following steps as detailed are critical and should be followed to ensure adequate preparation of the product.- The technetium Tc99m labeling reaction involved in the preparation of MYOVIEW Injection depends on maintaining tin in the divalent (reduced) state. Any oxidant present in the sodium pertechnetate Tc99m used may adversely affect the quality of the preparation. Sodium pertechnetate Tc99m containing oxidants should not be used for the preparation of the labeled product.
- Elute the technetium generator with sodium chloride injection, USP.
- Insert a venting needle (standard 18 to 26 gauge needle, not provided) through the rubber septum of the shielded vial containing the lyophilized powder.
- Inject no more than 8.8 GBq (240 mCi) of technetium Tc99m generator eluate into the shielded vial.
- Use sodium chloride injection, USP as a diluent. Inject 4 to 8 mL to achieve a radioactive concentration no greater than 1.1 GBq/mL (30 mCi/mL) in the vial.
- Before removing the syringe from the vial, withdraw 2 mL of gas from above the solution.
- Remove the venting needle.
- Mix gently for 10 seconds to ensure complete dissolution of the powder.
- Incubate at room temperature for 15 minutes.
- Assay the total activity using a suitably calibrated instrument; complete the user radiation label and attach it to the vial.
- Measure the pH of the prepared injection and verify it is between 7.5 to 9.0.
- Store the radiolabeled MYOVIEW vial at 2° to 25°C (36° to 77°F) and use radiolabeled injection within 12 hours of preparation.
)2.5 Determination of Radiochemical PurityObtain the following materials:- SA TLC strip (2 cm × 20 cm), do not heat activate
- Ascending chromatography tank and cover
- Mixture of acetone and dichloromethane (65:35% v/v), prepare freshly
- Syringe (1 mL) with needle (22 to 25 gauge)
- Suitable counting equipment
Perform the following:- Pour the 65:35% v/v acetone:dichloromethane mixture into the chromatography tank to a depth of 1 cm and cover the tank to allow the solvent vapor to equilibrate.
- Mark SA TLC strip with a pencil line at 3 cm from the bottom and, using an ink marker pen, at 15 cm from the pencil line. The pencil line indicates the origin where the sample is to be applied and movement of color from the ink line will indicate the position of the solvent front when upward elution should be stopped.
- Mark cutting positions at 3.75 cm and 12 cm above the origin [retention value (Rf) 0.25 and 0.8 respectively] in pencil.
- Using a 1 mL syringe and needle, apply a 10 microliter sample of the prepared injection at the origin of the strip. Do not allow the spot to dry. Place the strip in the chromatography tank immediately and replace the cover. Ensure that the strip is not adhering to the walls of the tank.
Note: A 10 microliter sample will produce a spot with a diameter of approximately 10 mm. Different sample volumes have been shown to give unreliable radiochemical purity values. - When the solvent reaches the ink line, remove the strip from the tank and allow it to dry.
- Cut the strip into 3 pieces at the marked cutting positions and measure the activity on each using suitable counting equipment. Ensure similar counting geometry for each piece and minimize equipment dead time losses. Note: Free Tc99m pertechnetate runs to the top piece of the strip. MYOVIEW runs to the center piece of the strip. Reduced hydrolyzed Tc99m and any hydrophilic complex impurities remain at the origin in the bottom piece of the strip.TLC strip diagram
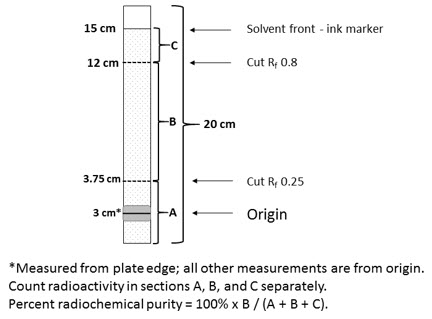
- Calculate the radiochemical purity from:
% Tc99m tetrofosmin = Activity of Center Piece (B) × 100 Total Activity of Three Pieces (A+B+C) - Do not use material if the radiochemical purity is less than 90%.

Image - Imaging may begin 15 minutes following administration of the agent ()
2.6 Imaging Instructions- Imaging may begin 15 minutes after injection.
- The recommended imaging duration of the scan may vary depending on dose, imaging acquisition, and reconstruction parameters.
Kit for the preparation of technetium Tc99m tetrofosmin injection: 10 mL multiple-dose, clear, glass vial with a white sterile, non-pyrogenic, lyophilized powder of 0.23 mg tetrofosmin, 0.03 mg stannous chloride dihydrate, 0.32 disodium sulphosalicylate, 1 mg sodium D-gluconate and 1.8 mg sodium hydrogen carbonate.
Following radiolabeling with the Tc99m eluate, MYOVIEW is a clear solution not exceeding 1,110 MBq/mL (30 mCi/mL) of Tc99m.
- Advise the pregnant woman of the potential risk to the fetus based on the radiation dose from technetium Tc99m and the gestational timing of exposure ()
8.1 PregnancyRisk SummaryThere are no data with technetium Tc99m tetrofosmin use in pregnant women to inform any drug associated risks. Animal reproduction studies with technetium Tc99m tetrofosmin have not been conducted. However, all radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. If considering technetium Tc99m tetrofosmin administration to a pregnant woman advise the pregnant woman of risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
- Lactation - A lactating woman should pump and discard breastmilk for 60 hours after technetium Tc99m tetrofosmin administration ()
8.2 LactationRisk SummaryTechnetium Tc99m tetrofosmin is present in human milk in small amounts (<1% of maternal dose). There are no data available regarding the effects of technetium Tc99m tetrofosmin on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for MYOVIEW and any potential adverse effects on the breastfed child from MYOVIEW or from the underlying maternal condition.
Clinical ConsiderationsTo decrease radiation exposure to the breastfed infant, advise a lactating woman to pump and discard breast milk for 60 hours (10 half-lives) after technetium Tc99m tetrofosmin administration.
None.
- Risk with exercise or pharmacologic stress:
- Continuous cardiac monitoring should be performed in studying patients with known or suspected coronary artery disease ()
5.1 Risks Associated with Exercise or Pharmacologic StressPatients evaluated with exercise or pharmacologic stress may experience serious adverse reactions such as myocardial infarction, arrhythmia, hypotension, bronchoconstriction, and cerebrovascular reactions such as headache, paraesthesias, convulsions, somnolence and cerebrovascular accident, including hemorrhage. Perform stress testing in the setting where cardiac resuscitation equipment and trained staff are readily available. When pharmacologic stress is selected as an alternative to exercise, perform the procedure in accordance with the pharmacologic stress agent's prescribing information.
- When pharmacologic stress is selected as an alternative to exercise, perform the procedure in accordance with the pharmacologic stress agent's prescribing information ()
5.1 Risks Associated with Exercise or Pharmacologic StressPatients evaluated with exercise or pharmacologic stress may experience serious adverse reactions such as myocardial infarction, arrhythmia, hypotension, bronchoconstriction, and cerebrovascular reactions such as headache, paraesthesias, convulsions, somnolence and cerebrovascular accident, including hemorrhage. Perform stress testing in the setting where cardiac resuscitation equipment and trained staff are readily available. When pharmacologic stress is selected as an alternative to exercise, perform the procedure in accordance with the pharmacologic stress agent's prescribing information.
- Continuous cardiac monitoring should be performed in studying patients with known or suspected coronary artery disease (
- Appropriate safety measures should be used to minimize radiation exposure to clinical personnel and to the patient consistent with proper patient management (,
2.1 Radiation Safety – Drug HandlingTechnetium Tc99m tetrofosmin is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure during administration
[see Warnings and Precautions (5.2)]. Use waterproof gloves and effective shielding, including syringe shields, when preparing and administering technetium Tc99m tetrofosmin injection.)5.2 Radiation RisksTechnetium Tc99m contributes to a patient's overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe handling and preparation radiolabeling procedures to protect patients and health care workers from unintentional radiation exposure. Encourage adequate hydration; instruct patients to void when the examination is completed and as often thereafter as possible
[see Dosage and Administration (2.1)and (2.3)].