Nalbuphine Hydrochloride Prescribing Information
Nalbuphine hydrochloride injection is indicated for the management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate. Nalbuphine hydrochloride injection can also be used as a supplement to balanced anesthesia, for preoperative and postoperative analgesia, and for obstetrical analgesia during labor and delivery.
Because of the risks of addiction, abuse, and misuse, with opioids, which can occur at any dosage or duration [see
- Have not been tolerated, or are not expected to be tolerated,
- Have not provided adequate analgesia or are not expected to provide adequate analgesia.
Nalbuphine hydrochloride injection should not be used for an extended period of time unless the pain remains severe enough to require an opioid analgesic and for which alternative treatment options continue to be inadequate.
Use the lowest effective dosage for the shortest duration of time consistent with individual patient treatment goals [see
There is variability in the opioid analgesic dose and duration needed to adequately manage pain due both to the cause of pain and to individual patient factors. Initiate the dosing regimen for each patient individually, taking into account the patient's underlying cause and severity of pain, prior analgesic treatment and response, and risk factors for addiction, abuse, and misuse [see
Respiratory depression can occur at any time during opioid therapy, especially when initiating and following dosage increases with nalbuphine hydrochloride injection. Consider this risk when selecting an initial dose and when making dose adjustments [see
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
The usual recommended adult dose is 10 mg for a 70 kg individual administered subcutaneously, intramuscularly, or intravenously; this dose may be repeated every 3 to 6 hours as necessary. Use the lowest dose necessary to achieve adequate analgesia. Dosage should be adjusted according to the severity of the pain, physical status of the patient, and other medications which the patient may be receiving (see
The use of nalbuphine hydrochloride injection as a supplement to balanced anesthesia requires larger doses than those recommended for analgesia. Induction doses of nalbuphine hydrochloride range from 0.3 mg/kg to 3 mg/kg intravenously to be administered over a 10-to-15-minute period with maintenance doses of 0.25 to 0.5 mg/kg in single intravenous administrations as required. The use of nalbuphine hydrochloride injection may be followed by respiratory depression which can be reversed with the opioid antagonist naloxone hydrochloride.
Titrate the dose based upon the individual patient's response to their initial dose of nalbuphine hydrochloride injection. Individually titrate nalbuphine hydrochloride injection to a dose that provides adequate analgesia and minimizes adverse reactions. Continually reevaluate patients receiving nalbuphine hydrochloride to assess the maintenance of pain control, signs and symptoms of opioid withdrawal, and other adverse reactions, as well as to reassess for the development of addiction, abuse, or misuse [see
If the level of pain increases after dosage stabilization, attempt to identify the source of increased pain before increasing the nalbuphine hydrochloride dosage. If after increasing the dosage, unacceptable opioid-related adverse reactions are observed (including an increase in pain after a dosage increase), consider reducing the dosage [see
When a patient who has been taking nalbuphine hydrochloride injection regularly and may be physically dependent no longer requires therapy with nalbuphine hydrochloride injection, taper the dose gradually, by 25% to 50% every 2 to 4 days, while regularly evaluating for signs and symptoms of withdrawal. If the patient develops these signs or symptoms, raise the dose to the previous level and taper more slowly, either by increasing the interval between decreases, decreasing the amount of change in dose, or both. Do not abruptly discontinue nalbuphine hydrochloride injection in a physically-dependent patient [see
Nalbuphine hydrochloride injection is contraindicated in patients with:
- Significant respiratory depression [see WARNINGS]
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment [see WARNINGS]
- Known or suspected gastrointestinal obstruction, including paralytic ileus [see WARNINGS]
- Hypersensitivity to nalbuphine to any of the other ingredients in nalbuphine hydrochloride injection.
Nalbuphine hydrochloride injection contains nalbuphine. As an opioid, nalbuphine exposes users to the risks of addiction, abuse, and misuse [see
Opioids are sought for non-medical use and are subject to diversion from legitimate prescribed use. Consider these risks when handling nalbuphine hydrochloride injection. Strategies to reduce these risks include proper product storage and control practices for a C-II drug. Contact local state professional licensing board or state- controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Serious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient's clinical status [see
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of nalbuphine hydrochloride injection, the risk is greatest during the initiation of therapy or following a dosage increase. Monitor patients closely for respiratory depression, especially within the first 24 to 72 hours of initiating therapy with and following dosage increases of nalbuphine hydrochloride injection.
To reduce the risk of respiratory depression, proper dosing and titration of nalbuphine hydrochloride injection are essential [see
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see
Profound sedation, respiratory depression, coma, and death may result from the concomitant use of nalbuphine hydrochloride injection with benzodiazepines and/or other CNS depressants, including alcohol (e.g., non- benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Monitor patients closely for signs and symptoms of respiratory depression and sedation.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see
If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Follow patients closely for signs and symptoms of respiratory depression and sedation.
Advise both patients and caregivers about the risks of respiratory depression and sedation when nalbuphine hydrochloride injection is used with benzodiazepines or other CNS depressants (including alcohol and illicit drugs). Advise patients not to drive or operate heavy machinery until the effects of concomitant use of the benzodiazepine or other CNS depressant have been determined. Screen patients for risk of substance use disorders, including opioid abuse and misuse, and warn them of the risk for overdose and death associated with the use of additional CNS depressants including alcohol and illicit drugs [see
Opioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. This condition differs from tolerance, which is the need for increasing doses of opioids to maintain a defined effect [see
Cases of OIH have been reported, both with short-term and longer-term use of opioid analgesics. Though the mechanism of OIH is not fully understood, multiple biochemical pathways have been implicated. Medical literature suggests a strong biologic plausibility between opioid analgesics and OIH and allodynia. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic or opioid rotation (safely switching the patient to a different opioid moiety) [see
The use of nalbuphine hydrochloride injection in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Monitor such patients closely, particularly when initiating and titrating nalbuphine hydrochloride injection and when nalbuphine hydrochloride injection is given concomitantly with other drugs that depress respiration [see
Cases of adrenal insufficiency have been reported with opioid use, more often following greater than 1 month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
Nalbuphine hydrochloride injection may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics)
In patients who may be susceptible to the intracranial effects of CO2 retention (e.g., those with evidence of increased intracranial pressure or brain tumors), nalbuphine hydrochloride injection may reduce respiratory drive, and the resultant CO2 retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with nalbuphine hydrochloride injection.
Opioids may also obscure the clinical course in a patient with a head injury. Avoid the use of nalbuphine hydrochloride injection in patients with impaired consciousness or coma.
Nalbuphine hydrochloride injection is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The nalbuphine in nalbuphine hydrochloride injection may cause spasm of the sphincter of Oddi. Opioids may cause increases in serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
The nalbuphine in nalbuphine hydrochloride injection may increase the frequency of seizures in patients with seizure disorders and may increase the risk of seizures occurring in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during nalbuphine hydrochloride injection therapy.
The use of nalbuphine hydrochloride injection, a mixed agonist/antagonist opioid analgesic, in patients who are receiving a full opioid agonist analgesic may reduce the analgesic effect and/or precipitate withdrawal symptoms. Avoid concomitant use of nalbuphine hydrochloride injection with a full opioid agonist analgesic.
When discontinuing
Nalbuphine hydrochloride injection may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of nalbuphine hydrochloride injection and know how they will react to the medication [see
Maintain patient under observation until recovered from nalbuphine hydrochloride injection effects that would affect driving or other potentially dangerous tasks.
Severe fetal bradycardia has been reported when nalbuphine hydrochloride injection is administered during labor. Naloxone may reverse these effects. Although there are no reports of fetal bradycardia earlier in pregnancy, it is possible that this may occur. Avoid the use of nalbuphine hydrochloride injection in pregnant women unless the potential benefit outweighs the risk to the fetus, and if appropriate measures such as fetal monitoring are taken to detect and manage any potential adverse effect on the fetus.
The placental transfer of nalbuphine is high, rapid, and variable with a maternal to fetal ratio ranging from 1:0.37 to 1:6. Fetal and neonatal adverse effects that have been reported following the administration of nalbuphine to the mother during labor include fetal bradycardia, respiratory depression at birth, apnea, cyanosis, and hypotonia. Some of these events have been life-threatening. Maternal administration of naloxone during labor has normalized these effects in some cases. Severe and prolonged fetal bradycardia has been reported. Permanent neurological damage attributed to fetal bradycardia has occurred. A sinusoidal fetal heart rate pattern associated with the use of nalbuphine has also been reported. Nalbuphine hydrochloride injection should be used during labor and delivery only if clearly indicated and only if the potential benefit outweighs the risk to the infant. Newborns should be monitored for respiratory depression, apnea, bradycardia and arrhythmias if nalbuphine hydrochloride injection has been used.
The most frequent adverse reaction in 1066 patients treated in clinical studies with nalbuphine hydrochloride injection was sedation 381 (36%).
Less frequent reactions were: sweaty/clammy 99 (9%), nausea/vomiting 68 (6%), dizziness/vertigo 58 (5%), dry mouth 44 (4%), and headache 27 (3%).
Other adverse reactions which occurred (reported incidence of 1% or less) were:
Dermatologic: Itching, burning, urticaria.
Miscellaneous: Speech difficulty, urinary urgency, blurred vision, flushing and warmth.
The following adverse reactions have been identified during post approval use of nalbuphine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Abdominal pain, pyrexia, depressed level or loss of consciousness, somnolence, tremor, anxiety, pulmonary edema, agitation, seizures, and injection site reactions such as pain, swelling, redness, burning, and hot sensations. Death has been reported from severe allergic reactions to nalbuphine hydrochloride injection treatment. Fetal death has been reported where mothers received nalbuphine hydrochloride injection during labor and delivery.
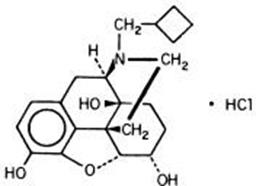
Nalbuphine hydrochloride injection is a synthetic opioid agonist-antagonist analgesic of the phenanthrene series. It is chemically related to both the widely used opioid antagonist, naloxone, and the potent opioid analgesic, oxymorphone. Chemically nalbuphine hydrochloride is 17-(cyclobutylmethyl)- 4,5α-epoxymorphinan-3,6α,14-triol hydrochloride. Nalbuphine hydrochloride molecular weight is 393.91 and is soluble in H2O (35.5 mg/mL @ 25ºC) and ethanol (0.8%); insoluble in CHCl3 and ether.
Nalbuphine hydrochloride has pKa values of 8.71 and 9.96. The molecular formula is C21H27NO4 · HCl. The structural formula is:
Nalbuphine hydrochloride injection is a sterile solution suitable for subcutaneous, intramuscular, or intravenous injection. Nalbuphine hydrochloride injection is available in two concentrations, 10 mg and 20 mg of nalbuphine hydrochloride injection per mL. Both strengths in 10 mL vials contain 0.94% sodium citrate dihydrate, 1.26% citric acid anhydrous, and 0.2% of a 9:1 mixture of methylparaben and propylparaben as preservatives; pH is adjusted, if necessary, to 3.0 to 4.5 with hydrochloric acid. The 10 mg/mL strength contains 0.2% sodium chloride.
Nalbuphine hydrochloride injection is also available in ampuls in a sterile, paraben-free formulation in two concentrations, 10 mg and 20 mg of nalbuphine hydrochloride per mL. One mL of each strength contains 0.94% sodium citrate dihydrate, and 1.26% citric acid anhydrous; pH is adjusted, if necessary, to 3.0 to 4.5 with hydrochloric acid. The 10 mg/mL strength contains 0.2% sodium chloride.