Naloxone Hydrochloride
Naloxone Hydrochloride Prescribing Information
Naloxone hydrochloride nasal spray is indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression.
Naloxone hydrochloride nasal spray is intended for immediate administration as emergency therapy in settings where opioids may be present.
Naloxone hydrochloride nasal spray is not a substitute for emergency medical care.
Naloxone hydrochloride nasal spray is supplied as a single-dose intranasal spray containing 4 mg of naloxone hydrochloride in 0.1 mL.
The limited available data on naloxone use in pregnant women are not sufficient to inform a drug-associated risk. However, there are clinical considerations
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Naloxone hydrochloride crosses the placenta, and may precipitate withdrawal in the fetus, as well as in the opioid-dependent mother
Naloxone hydrochloride was administered during organogenesis to mice and rats at subcutaneous doses up to 10 mg/kg/day (equivalent to 6-times and 12-times, respectively, a human dose of 8 mg (two naloxone hydrochloride nasal sprays) (based on body surface area comparison). These studies demonstrated no embryotoxic or teratogenic effects due to naloxone hydrochloride.
Pregnant female rats were administered 2 or 10 mg/kg naloxone subcutaneously from Gestation Day 15 to Postnatal day 21. There were no adverse effects on the offspring (up to 12-times a human dose of 8 mg/day (two naloxone hydrochloride nasal sprays) based on body surface area comparison).
Naloxone hydrochloride nasal spray is contraindicated in patients known to be hypersensitive to naloxone hydrochloride or to any of the other ingredients.
The following serious adverse reactions are discussed elsewhere in the labeling:
- Precipitation of Severe Opioid Withdrawal [see Warnings and Precautions ]
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The following adverse reactions were observed in a naloxone hydrochloride nasal spray clinical study.
In a pharmacokinetic study of 30 healthy adult volunteers exposed to one spray of naloxone hydrochloride nasal spray in one nostril or two sprays of naloxone hydrochloride nasal spray, one in each nostril, the most common adverse reactions were: increased blood pressure, constipation, toothache, muscle spasms, musculoskeletal pain, headache, nasal dryness, nasal edema, nasal congestion, nasal inflammation, rhinalgia, and xeroderma.
The following adverse reactions have been identified primarily during post-approval use of naloxone hydrochloride in the post-operative setting. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: Hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. Excessive doses of naloxone hydrochloride in post-operative patients have resulted in significant reversal of analgesia, and have caused agitation.
Abrupt reversal of opioid effects in persons who were physically dependent on opioids has precipitated an acute withdrawal syndrome. Signs and symptoms have included: body aches, fever, sweating, runny nose, sneezing, piloerection, yawning, weakness, shivering or trembling, nervousness, restlessness or irritability, diarrhea, nausea or vomiting, abdominal cramps, increased blood pressure, tachycardia. In some patients, there may be aggressive behavior upon abrupt reversal of an opioid overdose. In the neonate, opioid withdrawal signs and symptoms also included convulsions, excessive crying, and hyperactive reflexes.
Naloxone hydrochloride nasal spray is a pre-filled, single dose intranasal spray. Chemically, naloxone hydrochloride dihydrate is the hydrochloride salt of (5R,9R,13S,14S)-17-Allyl-3,14-dihydroxy-4,5-epoxymorphinan-6-on hydrochloride dihydrate with the following structure:
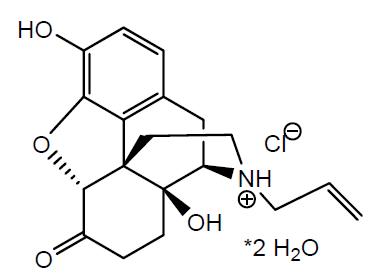
C19H22NO4Cl•2H2O M.W. 399.87
Naloxone hydrochloride, USP an opioid antagonist, occurs as a white to slightly off-white powder, and is freely soluble in water, soluble in ethanol (96%) and practically insoluble in toluene.
Each naloxone hydrochloride nasal spray contains a 4 mg single dose of naloxone hydrochloride, USP (equivalent to 3.6 mg of naloxone) in a 0.1 mL (100 microliter) aqueous solution.
Inactive ingredients include benzalkonium chloride (preservative), edetate disodium (stabilizer), sodium chloride, sodium hydroxide/hydrochloric acid to adjust pH, and purified water. The pH range is 3.5 to 5.5.