Nicardipine Hydrochloride
Nicardipine Hydrochloride Prescribing Information
Nicardipine hydrochloride capsules are indicated for the management of patients with chronic stable angina (effort-associated angina). Nicardipine hydrochloride capsules may be used alone or in combination with beta-blockers.
The dose should be individually titrated for each patient beginning with 20 mg three times daily. Doses in the range of 20 mg to 40 mg three times a day have been shown to be effective. At least 3 days should be allowed before increasing the nicardipine hydrochloride capsules dose to ensure achievement of steady-state plasma drug concentrations.
- Sublingual NTGmay be taken as required to abort acute anginal attacks during nicardipine hydrochloride capsules therapy.
- Prophylactic Nitrate Therapy:Nicardipine hydrochloride capsules may be safely co-administered with short- and long-acting nitrates.
- Beta-blockers: Nicardipine hydrochloride capsules may be safely co-administered with beta-blockers (see).
Drug InteractionsBeta BlockersIn controlled clinical studies, adrenergic beta-receptor blockers have been frequently administered concomitantly with nicardipine hydrochloride. The combination is well tolerated.
CimetidineCimetidine increases nicardipine hydrochloride plasma levels. Patients receiving the two drugs concomitantly should be carefully monitored.
DigoxinSome calcium blockers may increase the concentration of digitalis preparations in the blood. Nicardipine hydrochloride usually does not alter the plasma levels of digoxin; however, serum digoxin levels should be evaluated after concomitant therapy with nicardipine hydrochloride is initiated.
Maalox® 2Co-administration of Maalox TC had no effect on nicardipine hydrochloride absorption.
Fentanyl AnesthesiaSevere hypotension has been reported during fentanyl anesthesia with concomitant use of a beta-blocker and a calcium channel blocker. Even though such interactions were not seen during clinical studies with nicardipine hydrochloride, an increased volume of circulating fluids might be required if such an interaction were to occur.
CyclosporineConcomitant administration of oral or intravenous nicardipine and cyclosporine results in elevated plasma cyclosporine levels through nicardipine inhibition of hepatic microsomal enzymes, including CYP3A4. Plasma concentrations of cyclosporine should therefore be closely monitored, and its dosage reduced accordingly, in patients treated with nicardipine.
TacrolimusConcomitant administration of oral or intravenous nicardipine and tacrolimus may result in elevated plasma tacrolimus levels through nicardipine inhibition of hepatic microsomal enzymes, including CYP3A4. Closely monitor plasma concentrations of tacrolimus during nicardipine administration, and adjust the dose of tacrolimus accordingly.When therapeutic concentrations of furosemide, propranolol, dipyridamole, warfarin, quinidine or naproxen were added to human plasma (
in vitro), the plasma protein binding of nicardipine hydrochloride was not altered.
Nicardipine hydrochloride capsules are contraindicated in patients with hypersensitivity to the drug.
Because part of the effect of nicardipine hydrochloride capsules are secondary to reduced afterload, the drug is also contraindicated in patients with advanced aortic stenosis. Reduction of diastolic pressure in these patients may worsen rather than improve myocardial oxygen balance.
In multiple-dose US and foreign controlled short-term (up to 3 months) studies 1,910 patients received nicardipine hydrochloride alone or in combination with other drugs. In these studies adverse events were reported spontaneously; adverse experiences were generally not serious but occasionally required dosage adjustment and about 10% of patients left the studies prematurely because of them. Peak responses were not observed to be associated with adverse effects during clinical trials, but physicians should be aware that adverse effects associated with decreases in blood pressure (tachycardia, hypotension, etc.) could occur around the time of the peak effect. Most adverse effects were expected consequences of the vasodilator effects of nicardipine hydrochloride.
In controlled clinical studies, adrenergic beta-receptor blockers have been frequently administered concomitantly with nicardipine hydrochloride. The combination is well tolerated.
Nicardipine hydrochloride capsules for oral administration each contain 20 mg or 30 mg of nicardipine hydrochloride, USP. Nicardipine hydrochloride is a calcium ion influx inhibitor (slow channel blocker or calcium channel blocker).
Nicardipine hydrochloride is a dihydropyridine structure with the IUPAC (International Union of Pure and Applied Chemistry) chemical name 2-(benzyl-methyl amino)ethyl methyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate monohydrochloride, and it has the following structure:
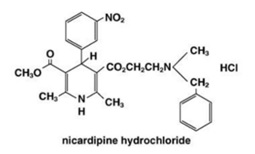
The molecular formula of nicardipine hydrochloride, USP is C26H29N3O6. HCl.
Nicardipine hydrochloride, USP is a pale greenish yellow, odorless, crystalline powder that melts at about 167°C to 171°C. It is soluble in methanol, sparingly soluble in ethanol, and slightly soluble in acetone, chloroform and water. It has a molecular weight of 515.99 g/mol.
Nicardipine hydrochloride capsules are available in hard gelatin capsules containing 20 mg or 30 mg nicardipine hydrochloride, USP with colloidal silicon dioxide, magnesium stearate, and pregelatinized maize starch as the inactive ingredients. The 20 mg strength is provided in blue opaque capsules, while the 30 mg capsules have light blue opaque cap and white opaque body. The capsule shell contains FD&C blue no. 1, FD&C red no. 3, D&C yellow no. 10, gelatin, and titanium dioxide. The capsules are printed with black ink composed of ammonia solution, black iron oxide, potassium hydroxide, propylene glycol, and shellac.