Nitroglycerin
Nitroglycerin Prescribing Information
Nitroglycerin ointment USP, 2% is indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of transdermal nitroglycerin is not sufficiently rapid for this product to be useful in aborting an acute anginal episode.
As noted above (
It is reasonable to believe that the rate and extent of nitroglycerin absorption from ointment may vary with the site and square measure of the skin over which a given dose of ointment is spread, but these relationships have not been adequately studied.
Controlled trials with other formulations of nitroglycerin have demonstrated that if plasma levels are maintained continuously, all anti-anginal efficacy is lost within 24 hours. This tolerance cannot be overcome by increasing the dose of nitroglycerin. As a result, any regimen of nitroglycerin ointment administration should include a daily nitrate-free interval. The minimum necessary length of such an interval has not been defined, but studies with other nitroglycerin formulations have shown that 10 to 12 hours is sufficient.
Thus, one appropriate dosing schedule for nitroglycerin ointment would begin with two daily 1/2- inch (7.5 mg) doses, one applied on rising in the morning and one applied six hours later. The dose could be doubled, and even doubled again, in patients tolerating this dose but failing to respond to it. The pouch is intended as a unit dose package only and is equivalent to approximately 1 inch as squeezed from the tube. Use entire contents of pouch to obtain full dose and discard immediately after use.
Each tube of ointment and each box of pouchs is supplied with a pad of ruled, impermeable, paper applicators. These applicators allow ointment to be absorbed through a much smaller area of skin than that used in any of the reported clinical trials, and the significance of this difference is not known. To apply the ointment using one of the applicators, place the applicator on a flat surface, printed side down. Squeeze the necessary amount of ointment from the tube onto the applicator, place the applicator (ointment side down) on the desired area of the skin, and tape the applicator into place.
Allergic reactions to organic nitrates are extremely rare, but they do occur. Nitroglycerin is contraindicated in patients who are allergic to it.
Adverse reactions to nitroglycerin are generally dose-related, and almost all of these reactions are the result of nitroglycerin's activity as a vasodilator. Headache, which may be severe, is the most commonly reported side effect. Headache may be recurrent with each daily dose, especially at higher doses. Transient episodes of lightheadedness, occasionally related to blood pressure changes, may also occur. Hypotension occurs infrequently, but in some patients it may be severe enough to warrant discontinuation of therapy. Syncope, crescendo angina, and rebound hypertension have been reported but are uncommon.
Allergic reactions to nitroglycerin are also uncommon, and the great majority of those reported have been cases of contact dermatitis or fixed drug eruptions in patients receiving nitroglycerin in ointments or patches. There have been a few reports of genuine anaphylactoid reactions, and these reactions can probably occur in patients receiving nitroglycerin by any route.
Extremely rarely, ordinary doses of organic nitrates have caused methemoglobinemia in normal-seeming patients; for further discussion of its diagnosis and treatment see OVERDOSAGE.
Data are not available to allow estimation of the frequency of adverse reactions during treatment with nitroglycerin ointment.
The vasodilating effects of nitroglycerin may be additive with those of other vasodilators. Alcohol, in particular, has been found to exhibit additive effects of this variety.
Marked symptomatic orthostatic hypotension has been reported when calcium channel blockers and organic nitrates were used in combination. Dose adjustments of either class of agents may be necessary.
Nitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is:
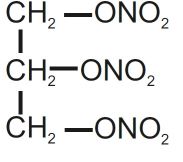
and whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both arteries and veins.
Nitroglycerin ointment USP, 2% for topical use contains lactose and 2% nitroglycerin in a base of lanolin, white petrolatum and purified water. Each inch (2.5 cm), as squeezed from the tube, contains approximately 15 mg of nitroglycerin.