Nystatin And Triamcinolone Acetonide
Nystatin And Triamcinolone Acetonide Prescribing Information
Nystatin and Triamcinolone Acetonide Ointment are indicated for the treatment of cutaneous candidiasis; it has been demonstrated that the nystatin-steroid combination provides greater benefit than the nystatin component alone during the first few days of treatment.
A thin film of Nystatin and Triamcinolone Acetonide Ointment is usually applied to the affected areas twice daily in the morning and evening. The preparation should be discontinued if symptoms persist after 25 days of therapy (see
Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria in some patients. Conditions that augment systemic absorption include application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings (see
Therefore, patients receiving a large dose of any potent topical steroid applied to a large surface area should be evaluated periodically for evidence of HPA axis suppression by using the urinary free cortisol and ACTH stimulation tests, and for impairment of internal homeostasis. If HPA axis suppression or elevation of the body temperature occurs, an attempt should be made to withdraw the drug, to reduce the frequency of application, or substitute a less potent steroid.
Recovery of HPA axis function and thermal homeostasis are generally prompt and complete upon discontinuation of the drug. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring supplemental systemic corticosteroids.
Children may absorb proportionally larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity (see
If irritation or hypersensitivity develops with the combination nystatin and triamcinolone acetonide, treatment should be discontinued and appropriate therapy instituted.
If there is a lack of therapeutic response, appropriate microbiological studies (e.g. KOH smears and/or cultures) should be repeated to confirm the diagnosis and rule out other pathogens, before instituting another course of therapy.
A urinary free cortisol test and ACTH stimulation test may be helpful in evaluating hypothalamic-pituitary-adrenal (HPA) axis suppression due to corticosteroids.
Nystatin and Triamcinolone Acetonide Ointment should not be used with occlusive dressings.
These preparations are contraindicated in those patients with a history of hypersensitivity to any of their components.
A single case (approximately one percent of patients studied) of acneiform eruption occurred with use of combined nystatin and triamcinolone acetonide in clinical studies.
Nystatin is virtually nontoxic and nonsensitizing and is well tolerated by all age groups, even during prolonged use. Rarely, irritation may occur.
The following local adverse reactions are reported infrequently with topical corticosteroids (reactions are listed in an approximate decreasing order of occurrence): burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, perioral secondary infection, skin atrophy, striae and miliaria.
Nystatin and Triamcinolone Acetonide Ointment, USP for dermatologic use contain the antifungal agent nystatin and the synthetic corticosteroid triamcinolone acetonide.
Nystatin, USP is a polyene antimycotic obtained from Streptomyces noursei. It is a yellow or slightly brownish powder hygroscopic with characteristics odor. It is freely soluble in dimethylformamide, slightly soluble in methanol, practically insoluble in water, alcohol and ether.
Structural formula:
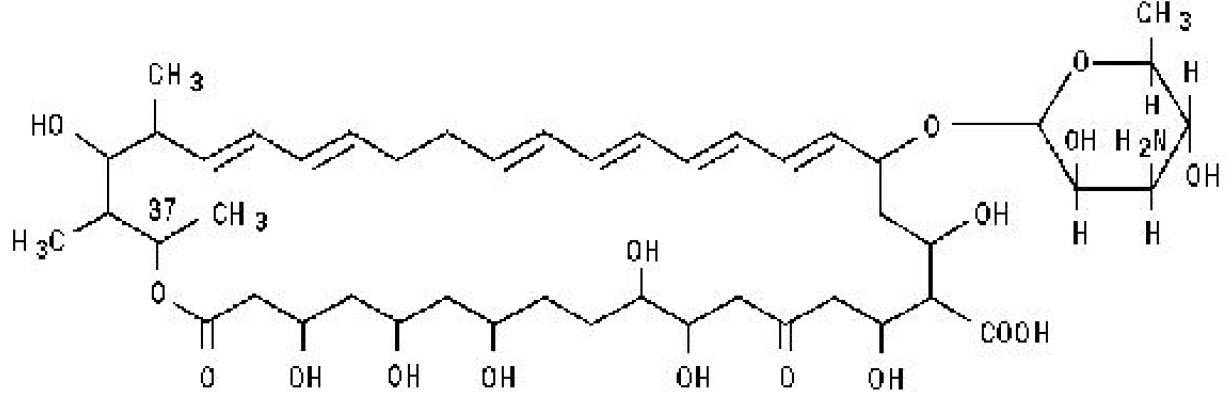
C47H75NO17 MW 926.13
Triamcinolone acetonide, USP is designated chemically as 9-fluoro-11ß, 16α, 17, 21- tetrahydroxypregna-1, 4-diene-3, 20-dione cyclic 16, 17-acetal with acetone.
It is a white to cream colored, crystalline powder, having not more than slight odor.
It is practically insoluble in water, sparingly soluble in dehydrated alcohol, in chloroform and in methanol.
Structural formula:
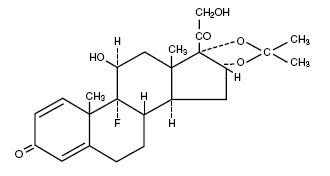
C24H31FO6 MW= 434.50
Each gram of Nystatin and Triamcinolone Acetonide Ointment provides 100,000 USP Nystatin units and 1 mg Triamcinolone Acetonide, USP in an ointment base of mineral oil and white petrolatum.
Nystatin exerts its antifungal activity against a variety of pathogenic and nonpathogenic yeasts and fungi by binding to sterols in the cell membrane. The binding process renders the cell membrane incapable of functioning as a selective barrier. Nystatin provides specific anticandidal activity to
Nystatin is not absorbed from intact skin or mucous membranes.