Octreoscan
(Indium In -111 Pentetreotide)Octreoscan Prescribing Information
Octreoscan, after radiolabeling, is an agent for the scintigraphic localization of primary and metastatic neuroendocrine tumors bearing somatostatin receptors.
Before administration, a patient should be well hydrated. After administration, the patient must be encouraged to drink fluids liberally. Elimination of extra fluid intake will help reduce the radiation dose by flushing out unbound, labelled pentetreotide by glomerular filtration. It is also recommended that a mild laxative (e.g., bisacodyl or lactulose) be given to the patient starting the evening before the radioactive drug is administered, and continuing for 48 hours. Ample fluid uptake is necessary during this period as a support both to renal elimination and the bowel-cleansing process. In a patient with an insulinoma, bowel-cleansing should be undertaken only after consultation with an endocrinologist.
The recommended intravenous dose for
The dose should be confirmed by a suitably calibrated radioactivity ionization chamber immediately before administration.
As with all intravenously administered products, Octreoscan should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Preparations containing particulate matter or discoloration should not be administered. They should be disposed of in a safe manner, in compliance with applicable regulations.
Aseptic techniques and effective shielding should be employed in withdrawing doses for administration to patients. Waterproof gloves should be worn during the administration procedure.
Do not administer Octreoscan in TPN solutions or through the same intravenous line.
None known.
The following adverse effects were observed in clinical trials at a frequency of less than 1% of 538 patients: dizziness, fever, flush, headache, hypotension, changes in liver enzymes, joint pain, nausea, sweating, and weakness. These adverse effects were transient. Also in clinical trials, there was one reported case of bradycardia and one case of decreased hematocrit and hemoglobin.
Pentetreotide is derived from octreotide which is used as a therapeutic agent to control symptoms from certain tumors. The usual dose for Indium In 111 Pentetreotide Injection is approximately 5 to 20 times less than for octreotide and is subtherapeutic. The following adverse reactions have been associated with octreotide in 3% to 10% of patients: nausea, injection site pain, diarrhea, abdominal pain/discomfort, loose stools, and vomiting. Hypertension and hyper- and hypoglycemia have also been reported with the use of octreotide.
The following adverse reactions have been identified during postapproval use of Octreoscan. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Octreoscan™ is a kit for the preparation of Indium In 111 Pentetreotide Injection, a radioactive diagnostic agent. It is a kit consisting of two components:
1) A 10-mL Octreoscan Reaction Vial which contains a lyophilized mixture of:
(i) 10 mcg pentetreotide [N-(diethylenetriamine-N,N,N′,N″-tetraacetic acid-N″-acetyl)-D-phenylalanyl-L-hemicystyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-L-hemicystyl-L-threoninol cyclic (2→7) disulfide], (also known as octreotide DTPA),
(ii) 2 mg gentisic acid [2, 5-dihydroxybenzoic acid],
(iii) 4.9 mg trisodium citrate, anhydrous,
(iv) 0.37 mg citric acid, anhydrous, and
(v) 10 mg inositol.
Pentetreotide has the following structural formula:
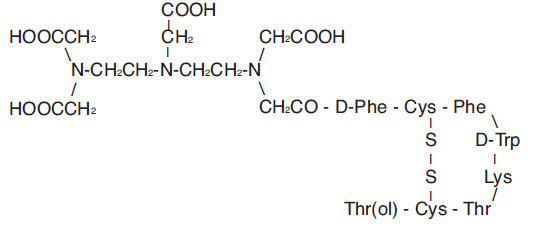
Prior to lyophilization, sodium hydroxide or hydrochloric acid may have been added for pH adjustment. The vial contents are sterile and nonpyrogenic. No bacteriostatic preservative is present.
2) A 10-mL vial of Indium In 111 Chloride Solution, which contains: 1.1 mL or 111 MBq/mL (3 mCi/mL) indium In 111 chloride in 0.02N HCl at time of calibration. The vial also contains ferric chloride at a concentration of 3.5 mcg/mL (ferric ion, 1.2 mcg/mL). The vial contents are sterile and nonpyrogenic. No bacteriostatic preservative is present.
Indium In 111 Pentetreotide Injection is prepared by combining the two kit components (
The Indium In 111 Pentetreotide Injection is suitable for intravenous administration as is, or it may be diluted to a maximum volume of 3 mL with 0.9% Sodium Chloride Injection, USP, immediately before intravenous administration. In either case, the radiolabeling yield of Indium In 111 Pentetreotide Injection should be determined before administration to the patient. A method recommended for determining the radiolabeling yield is presented at the end of this package insert (see
Pentetreotide is a DTPA conjugate of octreotide, which is a long-acting analog of the human hormone, somatostatin. Indium In 111 pentetreotide binds to somatostatin receptors on cell surfaces throughout the body. Within an hour of injection, most of the dose of indium In 111 pentetreotide distributes from plasma to extravascular body tissues and concentrates in tumors containing a high density of somatostatin receptors. After background clearance, visualization of somatostatin receptor-rich tissue is achieved. In addition to somatostatin receptor-rich tumors, the normal pituitary gland, thyroid gland, liver, spleen and urinary bladder also are visualized in most patients, as is the bowel, to a lesser extent. Excretion is almost exclusively via the kidneys.