Ocuflox
(Ofloxacin)Ocuflox Prescribing Information
CONJUNCTIVITIS: | |
| Gram-positive bacteria: | Gram-negative bacteria: |
Staphylococcus aureus | Enterobacter cloacae |
Staphylococcus epidermidis | Haemophilus influenzae |
Streptococcus pneumoniae | Proteus mirabilis |
Pseudomonas aeruginosa | |
CORNEAL ULCERS: | |
| Gram-positive bacteria: | Gram-negative bacteria: |
Staphylococcus aureus | Pseudomonas aeruginosa |
Staphylococcus epidermidis | Serratia marcescens* |
Streptococcus pneumoniae | Anaerobic species: |
| Propionibacterium acnes |
*Efficacy for this organism was studied in fewer than 10 infections
The recommended dosage regimen for the treatment of
| Days 1 and 2 | Instill one to two drops every two to four hours in the affected eye(s). |
| Days 3 through 7 | Instill one to two drops four times daily. |
| The recommended dosage regimen for the treatment of bacterial corneal ulcer is: | |
| Days 1 and 2 | Instill one to two drops into the affected eye every 30 minutes, while awake. Awaken at approximately four and six hours after retiring and instill one to two drops. |
| Days 3 through 7 to 9 | Instill one to two drops hourly, while awake. |
| Days 7 to 9 through | |
| treatment completion | Instill one to two drops, four times daily. |
There are rare reports of anaphylactic reaction/shock and fatal hypersensitivity reactions in patients receiving systemic quinolones, some following the first dose, including ofloxacin. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal or facial edema), airway obstruction, dyspnea, urticaria, and itching. A rare occurrence of Stevens-Johnson syndrome, which progressed to toxic epidermal necrolysis, has been reported in a patient who was receiving topical ophthalmic ofloxacin. If an allergic reaction to ofloxacin occurs, discontinue the drug. Serious acute hypersensitivity reactions may require immediate emergency treatment. Oxygen and airway management, including intubation should be administered as clinically indicated.
The most frequently reported drug-related adverse reaction was transient ocular burning or discomfort. Other reported reactions include stinging, redness, itching, chemical conjunctivitis/keratitis, ocular/periocular/facial edema, foreign body sensation, photophobia, blurred vision, tearing, dryness, and eye pain. Rare reports of dizziness and nausea have been received.
Refer to Warnings for additional adverse reactions.
Specific drug interaction studies have not been conducted with
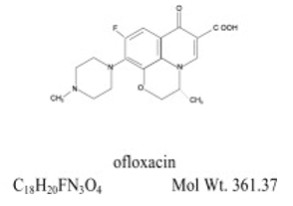
(±)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7