Ofloxacin
Ofloxacin Prescribing Information
Ofloxacin otic solution 0.3% is indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in the specific conditions listed below:
- For pediatric patients (from 6 months to 13 years old): Five drops (0.25 mL, 0.75 mg ofloxacin) instilled into the affected ear once daily for seven days.
- For patients 13 years and older: Ten drops (0.5 mL, 1.5 mg ofloxacin) instilled into the affected ear once daily for seven days.
- The solution should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness which may result from the instillation of a cold solution. The patient should lie with the affected ear upward, and then the drops should be instilled. This position should be maintained for five minutes to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear.
Five drops (0.25 mL, 0.75 mg ofloxacin) instilled into the affected ear twice daily for ten days. The solution should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness that may result from the instillation of a cold solution. The patient should lie with the affected ear upward, and then the drops should be instilled. The tragus should then be pumped 4 times by pushing inward to facilitate penetration of the drops into the middle ear. This position should be maintained for five minutes. Repeat, if necessary, for the opposite ear.
Ten drops (0.5 mL, 1.5 mg ofloxacin) instilled into the affected ear twice daily for fourteen days. The solution should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness that may result from the instillation of a cold solution. The patient should lie with the affected ear upward, before instilling the drops. The tragus should then be pumped 4 times by pushing inward to facilitate penetration into the middle ear. This position should be maintained for five minutes. Repeat, if necessary, for the opposite ear.
Ofloxacin otic solution 0.3% is contraindicated in patients with a history of hypersensitivity to ofloxacin, to other quinolones, or to any of the components in this medication.
In the Phase III clinical trials performed in support of once-daily dosing, 799 subjects with otitis externa and intact tympanic membranes were treated with ofloxacin otic solution. The studies, which served as the basis for approval, were 020 (pediatric, adolescents and adults), 016 (adolescents and adults) and 017 (pediatric). The following treatment-related adverse events occurred in 2 or more of the subjects:
†Studies 002/003 (BID) and 016/017 (QD) were active-controlled and comparative. | |||
| Adverse Event | Incidence Rate | ||
| Studies 002/003† BID (N=229) | Studies 016/017† QD (N=310) | Studies 020† QD (N=489) | |
| Application Site | 3% | 16.8% | 0.6% |
| Reaction | |||
| Pruritus | 4% | 1.2% | 1.0% |
| Earache | 1% | 0.6% | 0.8% |
| Dizziness | 1% | 0.0% | 0.6% |
| Headache | 0% | 0.3% | 0.2% |
| Vertigo | 1% | 0.0% | 0.0% |
Study 020 (QD) was open and non-comparative.
An unexpected increased incidence of application site reaction was seen in studies 016/017 and was similar for both ofloxacin and the active control drug (neomycin-polymyxin B sulfate- hydrocortisone). This finding is believed to be the result of specific questioning of the subjects regarding the incidence of application site reactions.
In once daily dosing studies, there were also single reports of nausea, seborrhea, transient loss of hearing, tinnitus, otitis externa, otitis media, tremor, hypertension and fungal infection.
In twice daily dosing studies, the following treatment-related adverse events were each reported in a single subject: dermatitis, eczema, erythematous rash, follicular rash, hypoaesthesia, tinnitus, dyspepsia, hot flushes, flushing and otorrhagia.
In Phase III clinical trials which formed the basis for approval, the following treatment-related adverse events occurred in 1% or more of the 656 subjects with non-intact tympanic membranes in AOM TT or CSOM treated twice-daily with ofloxacin otic solution:
| Adverse Event | Incidence (N=656) |
| Taste Perversion | 7% |
| Earache | 1% |
| Pruritus | 1% |
| Paraesthesia | 1% |
| Rash | 1% |
| Dizziness | 1% |
Other treatment-related adverse reactions reported in subjects with non-intact tympanic membranes included: diarrhea (0.6%), nausea (0.3%), vomiting (0.3%), dry mouth (0.5%), headache (0.3%), vertigo (0.5%), otorrhagia (0.6%), tinnitus (0.3%), fever (0.3%). The following treatment-related adverse events were each reported in a single subject: application site reaction, otitis externa, urticaria, abdominal pain, dysaesthesia, hyperkinesia, halitosis, inflammation, pain, insomnia, coughing, pharyngitis, rhinitis, sinusitis, and tachycardia.
Cases of uncommon transient neuropsychiatric disturbances have been included in spontaneous post- marketing reports. A causal relationship with ofloxacin otic solution 0.3% is unknown.
Ofloxacin otic solution 0.3% is a sterile aqueous anti-infective (anti-bacterial) solution for otic use. Chemically, ofloxacin has three condensed 6-membered rings made up of a fluorinated carboxyquinolone with a benzoxazine ring. The chemical name of ofloxacin is: (±)-9- fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid. The empirical formula of ofloxacin is C18H20FN3O4 and its molecular weight is 361.38. The structural formula is:
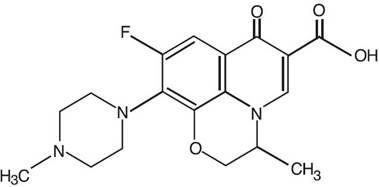
Ofloxacin otic contains 0.3% (3mg/mL) ofloxacin with benzalkonium chloride (0.0025%), sodium chloride (0.9%), and water for injection. Hydrochloric acid and sodium hydroxide are added to adjust the pH between 6.0 and 6.6.
Ofloxacin has been shown to be active against most isolates of the following microorganisms, both