Ondansetron
Ondansetron Prescribing Information
Warnings and Precautions, Myocardial Ischemia ( Myocardial ischemia has been reported in patients treated with ondansetron. In some cases, predominantly during intravenous administration, the symptoms appeared immediately after administration but resolved with prompt treatment. Coronary artery spasm appears to be the most common underlying cause. Therefore, monitor or advise patients for signs or symptoms of myocardial ischemia after oral administration of ondansetron [see Adverse Reactions (6.2)]. |
10/2021 |
Ondansetron is indicated for the prevention of nausea and vomiting associated with:
- highly emetogenic cancer chemotherapy, including cisplatin greater than or equal to 50 mg/m
2 - initial and repeat courses of moderately emetogenic cancer chemotherapy
- radiotherapy in patients receiving either total body irradiation, single high-dose fraction to the abdomen, or daily fractions to the abdomen
Ondansetron is also indicated for the prevention of postoperative nausea and/or vomiting.
Ondansetron hydrochloride tablets are white, round, film-coated tablets and are available in the following strengths:
- 4 mg – tablet debossed with “CE” on one side and “6” on the other side.
- 8 mg – tablet debossed with “CE” on one side and “7” on the other side.
Ondansetron is contraindicated in patients:
- known to have hypersensitivity (e.g., anaphylaxis) to ondansetron or any of the components of the formulation [see]
6.2 Postmarketing ExperienceThe following adverse reactions have been identified during postapproval use of ondansetron. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
CardiovascularArrhythmias (including ventricular and supraventricular tachycardia, premature ventricular contractions, and atrial fibrillation), bradycardia, electrocardiographic alterations (including second-degree heart block, QT/QTc interval prolongation, and ST segment depression), palpitations, and syncope. Rarely and predominantly with intravenous ondansetron, transient ECG changes, including QT interval prolongation have been reported.
Myocardial ischemia was reported predominately with intravenous administration
[see Warnings and Precautions (5.4)].GeneralFlushing: Rare cases of hypersensitivity reactions, sometimes severe (e.g., anaphylactic reactions, angioedema, bronchospasm, shortness of breath, hypotension, laryngeal edema, stridor) have also been reported. Laryngospasm, shock, and cardiopulmonary arrest have occurred during allergic reactions in patients receiving injectable ondansetron.HepatobiliaryLiver enzyme abnormalities.
Lower RespiratoryHiccups.
NeurologyOculogyric crisis, appearing alone, as well as with other dystonic reactions.
SkinUrticaria, Stevens-Johnson syndrome, and toxic epidermal necrolysis.
Eye DisordersCases of transient blindness, predominantly during intravenous administration, have been reported. These cases of transient blindness were reported to resolve within a few minutes up to 48 hours.
- receiving concomitant apomorphine due to the risk of profound hypotension and loss of consciousness
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see]
5.1 Hypersensitivity ReactionsHypersensitivity reactions, including anaphylaxis and bronchospasm, have been reported in patients who have exhibited hypersensitivity to other selective 5-HT3receptor antagonists. If hypersensitivity reactions occur, discontinue use of ondansetron; treat promptly per standard of care and monitor until signs and symptoms resolve
[see Contraindications (4)]. - QT Prolongation [see]
5.2 QT ProlongationElectrocardiogram (ECG) changes, including QT interval prolongation have been seen in patients receiving ondansetron. In addition, postmarketing cases of Torsade de Pointes have been reported in patients using ondansetron. Avoid ondansetron in patients with congenital long QT syndrome. ECG monitoring is recommended in patients with electrolyte abnormalities (e.g., hypokalemia or hypomagnesemia), congestive heart failure, bradyarrhythmias, or patients taking other medicinal products that lead to QT prolongation
[see Clinical Pharmacology (12.2)]. - Serotonin Syndrome [see]
5.3 Serotonin SyndromeThe development of serotonin syndrome has been reported with 5-HT3receptor antagonists alone. Most reports have been associated with concomitant use of serotonergic drugs (e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors, mirtazapine, fentanyl, lithium, tramadol, and intravenous methylene blue). Some of the reported cases were fatal. Serotonin syndrome occurring with overdose of ondansetron alone has also been reported. The majority of reports of serotonin syndrome related to 5-HT3receptor antagonist use occurred in a post-anesthesia care unit or an infusion center.
Symptoms associated with serotonin syndrome may include the following combination of signs and symptoms: mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, with or without gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome, especially with concomitant use of ondansetron and other serotonergic drugs. If symptoms of serotonin syndrome occur, discontinue ondansetron and initiate supportive treatment. Patients should be informed of the increased risk of serotonin syndrome, especially if ondansetron is used concomitantly with other serotonergic drugs
[see Drug Interactions (7.1), Overdosage (10)]. - Myocardial Ischemia [see]
5.4 Myocardial IschemiaMyocardial ischemia has been reported in patients treated with ondansetron. In some cases, predominantly during intravenous administration, the symptoms appeared immediately after administration but resolved with prompt treatment. Coronary artery spasm appears to be the most common underlying cause. Therefore, monitor or advise patients for signs or symptoms of myocardial ischemia after oral administration of ondansetron[see Adverse Reactions (6.2)]. - Masking of Progressive Ileus and Gastric Distension [see]
5.5 Masking of Progressive Ileus and Gastric DistensionThe use of ondansetron in patients following abdominal surgery or in patients with chemotherapy-induced nausea and vomiting may mask a progressive ileus and/or gastric distension. Monitor for decreased bowel activity, particularly in patients with risk factors for gastrointestinal obstruction.
ondansetron is not a drug that stimulates gastric or intestinal peristalsis. It should not be used instead of nasogastric suction.
The active ingredient in ondansetron tablets is ondansetron hydrochloride as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT
3 receptor type. Chemically it is (±) 1, 2, 3, 9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one, monohydrochloride, dihydrate. It has the following structural formula:
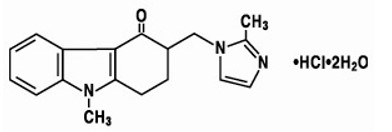
The empirical formula is C18H19N3O•HCl•2H2O, representing a molecular weight of 365.9 g/mol.
Ondansetron hydrochloride dihydrate is a white to off-white powder that is soluble in water and normal saline.
Each 4-mg ondansetron tablet for oral administration contains ondansetron hydrochloride dihydrate equivalent to 4 mg of ondansetron. Each 8-mg ondansetron tablet for oral administration contains ondansetron hydrochloride dihydrate equivalent to 8 mg of ondansetron. Each tablet also contains the inactive ingredients lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch, polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, and lecithin.