Oxacillin
(Oxacillin Sodium)Oxacillin Prescribing Information
Oxacillin is indicated in the treatment of infections caused by penicillinase producing staphylococci which have demonstrated susceptibility to the drug. Cultures and susceptibility tests should be performed initially to determine the causative organism and its susceptibility to the drug (see
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
Oxacillin may be used to initiate therapy in suspected cases of resistant staphylococcal infections prior to the availability of susceptibility test results. Oxacillin should not be used in infections caused by organisms susceptible to penicillin G. If the susceptibility tests indicate that the infection is due to an organism other than a resistant
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Oxacillin for Injection, USP and other antibacterial drugs, Oxacillin for Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Bacteriologic studies to determine the causative organisms and their susceptibility to oxacillin should always be performed. Duration of therapy varies with the type of severity of infection as well as the overall condition of the patient; therefore, it should be determined by the clinical and bacteriological response of the patient. In severe staphylococcal infections, therapy with oxacillin should be continued for at least 14 days. Therapy should be continued for at least 48 hours after the patient has become afebrile, asymptomatic, and cultures are negative. Treatment of endocarditis and osteomyelitis may require a longer duration of therapy.
With intravenous administration, particularly in elderly patients, care should be taken because of the possibility of thrombophlebitis.
Drug | Adults | Infants and Children <40 kg (88 lbs) | Other Recommendations |
| Oxacillin | 250 to 500 mg IV every 4 to 6 hours (mild to moderate infections) | 50 mg/kg/day IV in equally divided doses every 6 hours (mild to moderate infections) | |
| Oxacillin | 1 gram IV every 4 to 6 hours (severe infections) | 100 mg/kg/day IV in equally divided doses every 4 to 6 hours (severe infections) | Premature and Neonates 25 mg/kg/day IV |
A history of a hypersensitivity (anaphylactic) reaction to any penicillin is a contraindication.
The reported incidence of allergic reactions to penicillin ranges from 0.7 to 10 percent (see
Serious and occasionally fatal hypersensitivity (anaphylactic shock with collapse) reactions have occurred in patients receiving penicillin. The incidence of anaphylactic shock in all penicillin-treated patients is between 0.015 and 0.04 percent. Anaphylactic shock resulting in death has occurred in approximately 0.002 percent of the patients treated.
When oxacillin therapy is indicated, it should be initiated only after a comprehensive patient drug and allergy history has been obtained. If an allergic reaction occurs, oxacillin should be discontinued and appropriate therapy instituted.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against
Two types of allergic reactions to penicillins are noted clinically, immediate and delayed.
Immediate reactions usually occur within 20 minutes of administration and range in severity from urticaria and pruritus to angioneurotic edema, laryngospasm, bronchospasm, hypotension, vascular collapse and death. Such immediate anaphylactic reactions are very rare (see
Serious and occasionally fatal hypersensitivity (anaphylactic shock with collapse) reactions have occurred in patients receiving penicillin. The incidence of anaphylactic shock in all penicillin-treated patients is between 0.015 and 0.04 percent. Anaphylactic shock resulting in death has occurred in approximately 0.002 percent of the patients treated.
When oxacillin therapy is indicated, it should be initiated only after a comprehensive patient drug and allergy history has been obtained. If an allergic reaction occurs, oxacillin should be discontinued and appropriate therapy instituted.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against
Delayed allergic reactions to penicillin therapy usually occur after 48 hours and sometimes as late as 2 to 4 weeks after initiation of therapy.
Manifestations of this type of reaction include serum sickness-like symptoms (i.e.
Tetracycline, a bacteriostatic antibiotic, may antagonize the bactericidal effect of penicillin and concurrent use of these drugs should be avoided.
Oxacillin blood levels may be increased and prolonged by concurrent administration of probenecid which blocks the renal tubular secretion of penicillins. Probenecid decreases the apparent volume of distribution and slows the rate of excretion by competitively inhibiting renal tubular secretion of penicillins.
Oxacillin-probenecid therapy should be limited to those infections where very high serum levels of oxacillin are necessary.
Oxacillin for Injection, USP is a semisynthetic penicillin antibiotic derived from the penicillin nucleus, 6-amino-penicillanic acid. It is the sodium salt in parenteral dosage form. Each Pharmacy Bulk Package bottle of Oxacillin for Injection, USP contains oxacillin sodium monohydrate equivalent to 10 grams of oxacillin. The sodium content is 63.77 mg [2.77 mEq] per gram oxacillin. The product is buffered with 20 mg dibasic sodium phosphate per gram oxacillin. Oxacillin for Injection, USP is a white to off-white powder filled in clear glass bottles. Dilute solutions are essentially clear and colorless to yellow.
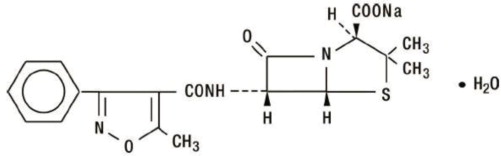
The chemical name of oxacillin sodium is 4-Thia-1-azabicyclo [3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-6-[[(5-methyl-3-phenyl-4-isoxazolyl) carbonyl] amino]-7-oxo-monosodium salt, monohydrate, [2S(2α, 5α, 6β)]. It is resistant to inactivation by the enzyme penicillinase (beta-lactamase). The molecular formula of oxacillin sodium is C19H18N3NaO5S•H2O. The molecular weight is 441.43.
A pharmacy bulk package is a container of a sterile preparation for parenteral use that contains many single doses. The contents of this pharmacy bulk package are intended for use by a pharmacy admixture service for addition to suitable parenteral fluids in the preparation of admixtures for intravenous infusion (see
- The container closure may be penetrated only one time after reconstitution, utilizing a suitable sterile dispensing set which allows measured distribution of the contents.
- Use of this product is restricted to a suitable work area, such as a laminar flow hood.
- Once this container closure has been punctured, withdrawal of the contents should be completed without delay. If prompt fluid transfer cannot be accomplished, discard the contents no later than 4 HOURS after initial closure puncture. This time limit should begin with the introduction of solvent for diluent into the Pharmacy Bulk Package.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Do not add supplementary medication to Oxacillin for Injection, USP.