Oxazepam Prescribing Information
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe oxazepam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of oxazepam than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking oxazepam, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when oxazepam is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see
Before prescribing Oxazepam and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of Oxazepam, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of Oxazepam along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
The continued use of benzodiazepines, including Oxazepam, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of Oxazepam after continued use or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life threatening. (e.g., seizures) (see
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see
As with other CNS-acting drugs, patients should be cautioned against driving automobiles or operating dangerous machinery until it is known that they do not become drowsy or dizzy on oxazepam therapy.
Patients should be warned that the effects of alcohol or other CNS-depressant drugs may be additive to those of Oxazepam, possibly requiring adjustment of dosage or elimination of such agents.
Although hypotension has occurred only rarely, oxazepam should be administered with caution to patients in whom a drop in blood pressure might lead to cardiac complications. This is particularly true in the elderly patient.
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
Advise both patients and caregivers about the risks of potentially fatal respiratory depression and sedation when Oxazepam is used with opioids and not to use such drugs concomitantly unless supervised by a health care provider. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see
Inform patients that the use of Oxazepam, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug (see
Inform patients that the continued use of Oxazepam may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of Oxazepam may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of Oxazepam may require a slow taper (see
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid- related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and monitor patients closely for respiratory depression and sedation.
Safety and effectiveness in pediatric patients under 6 years of age have not been established. Absolute dosage for pediatric patients 6 to 12 years of age is not established.
Clinical studies of oxazepam were not adequate to determine whether subjects aged 65 and over respond differently than younger subjects. Age (<80 years old) does not appear to have a clinically significant effect on oxazepam kinetics [see
Clinical circumstances, some of which may be more common in the elderly, such as hepatic or renal impairment, should be considered. Greater sensitivity of some older individuals to the effects of oxazepam (e.g., sedation, hypotension, paradoxical excitation) cannot be ruled out
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe oxazepam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of oxazepam than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking oxazepam, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when oxazepam is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see
Before prescribing Oxazepam and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of Oxazepam, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of Oxazepam along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
The continued use of benzodiazepines, including Oxazepam, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of Oxazepam after continued use or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life threatening. (e.g., seizures) (see
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see
As with other CNS-acting drugs, patients should be cautioned against driving automobiles or operating dangerous machinery until it is known that they do not become drowsy or dizzy on oxazepam therapy.
Patients should be warned that the effects of alcohol or other CNS-depressant drugs may be additive to those of Oxazepam, possibly requiring adjustment of dosage or elimination of such agents.
Because of the flexibility of this product and the range of emotional disturbances responsive to it, dosage should be individualized for maximum beneficial effects.
| OXAZEPAM | Usual Dose |
| Mild-to-moderate anxiety, with associated tension, irritability, agitation, or related symptoms of functional origin secondary to organic | 10 to 15 mg. 3 or 4 times daily |
| Severe anxiety syndromes, agitation, or anxiety associated with depression | 15 to 30 mg. 3 or 4 times daily |
| Older patients with anxiety, tension, irritability, and agitation | Initial dosage: 10 mg, 3 times daily. If necessary, increase cautiously to 15 mg, 3 or 4 times daily. |
| Alcoholics with acute inebriation, tremulousness, or anxiety on withdrawal | 15 to 30 mg, 3 or 4 times daily |
This product is not indicated in pediatric patients under 6 years of age. Absolute dosage for pediatric patients 6 to 12 years of age is not established.
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Oxazepam or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe oxazepam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of oxazepam than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking oxazepam, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when oxazepam is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see
Before prescribing Oxazepam and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of Oxazepam, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of Oxazepam along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
The continued use of benzodiazepines, including Oxazepam, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of Oxazepam after continued use or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life threatening. (e.g., seizures) (see
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see
As with other CNS-acting drugs, patients should be cautioned against driving automobiles or operating dangerous machinery until it is known that they do not become drowsy or dizzy on oxazepam therapy.
Patients should be warned that the effects of alcohol or other CNS-depressant drugs may be additive to those of Oxazepam, possibly requiring adjustment of dosage or elimination of such agents.
Because of the flexibility of this product and the range of emotional disturbances responsive to it, dosage should be individualized for maximum beneficial effects.
| OXAZEPAM | Usual Dose |
| Mild-to-moderate anxiety, with associated tension, irritability, agitation, or related symptoms of functional origin secondary to organic | 10 to 15 mg. 3 or 4 times daily |
| Severe anxiety syndromes, agitation, or anxiety associated with depression | 15 to 30 mg. 3 or 4 times daily |
| Older patients with anxiety, tension, irritability, and agitation | Initial dosage: 10 mg, 3 times daily. If necessary, increase cautiously to 15 mg, 3 or 4 times daily. |
| Alcoholics with acute inebriation, tremulousness, or anxiety on withdrawal | 15 to 30 mg, 3 or 4 times daily |
This product is not indicated in pediatric patients under 6 years of age. Absolute dosage for pediatric patients 6 to 12 years of age is not established.
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Oxazepam or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe oxazepam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of oxazepam than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking oxazepam, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when oxazepam is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see
Before prescribing Oxazepam and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of Oxazepam, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of Oxazepam along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
The continued use of benzodiazepines, including Oxazepam, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of Oxazepam after continued use or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life threatening. (e.g., seizures) (see
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see
As with other CNS-acting drugs, patients should be cautioned against driving automobiles or operating dangerous machinery until it is known that they do not become drowsy or dizzy on oxazepam therapy.
Patients should be warned that the effects of alcohol or other CNS-depressant drugs may be additive to those of Oxazepam, possibly requiring adjustment of dosage or elimination of such agents.
Oxazepam may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug.
Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use (see
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Oxazepam or reduce the dosage (see
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
Tolerance
Tolerance to Oxazepam may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of Oxazepam may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
History of previous hypersensitivity reaction to oxazepam. Oxazepam is not indicated in psychoses.
The necessity for discontinuation of therapy due to undesirable effects has been rare. Transient, mild drowsiness is commonly seen in the first few days of therapy. If it persists, the dosage should be reduced. In few instances, dizziness, vertigo, headache, and rarely syncope have occurred either alone or together with drowsiness. Mild paradoxical reactions, i.e., excitement, stimulation of affect, have been reported in psychiatric patients; these reactions may be secondary to relief of anxiety and usually appear in the first two weeks of therapy.
Other side effects occurring during oxazepam therapy include rare instances of minor diffuse skin rashes-morbilliform, urticarial, and maculopapular nausea, lethargy, edema, slurred speech, tremor and altered libido. Such side effects have been infrequent and are generally controlled with reduction of dosage. A case of an extensive fixed drug eruption also has been reported.
Although rare, leukopenia and hepatic dysfunction including jaundice have been reported during therapy. Periodic blood counts and liver-function tests are advisable. Ataxia with oxazepam has been reported in rare instances and does not appear to be specifically related to dose or age.
Although the following side reactions have not as yet been reported with oxazepam, they have occurred with related compounds (chlordiazepoxide and diazepam): paradoxical excitation with severe rage reactions, hallucinations, menstrual irregularities, change in EEG pattern, blood dyscrasias including agranulocytosis, blurred vision, diplopia, incontinence, stupor, disorientation, fever, and euphoria.
Transient amnesia or memory impairment has been reported in association with the use of benzodiazepines.
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid- related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and monitor patients closely for respiratory depression and sedation.
Oxazepam, USP is the first of a chemical series of compounds known as the 3- hydroxybenzodiazepinones. A therapeutic agent providing versatility and flexibility in control of common emotional disturbances, this product exerts prompt action in a wide variety of disorders associated with anxiety, tension, agitation, and irritability, and anxiety associated with depression. In tolerance and toxicity studies on several animal species, this product reveals significantly greater safety factors than related compounds (chlordiazepoxide and diazepam) and manifests a wide separation of effective doses and doses inducing side effects.
Oxazepam, is 7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-2
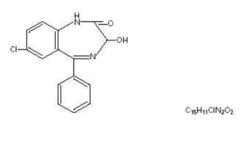 MW 286.72
MW 286.72
Oxazepam is a white-crystalline powder.
Each capsule for oral administration contain 10 mg, 15 mg or 30 mg of oxazepam.
Inactive ingredients: croscarmellose sodium, D&C yellow #10, FD&C blue #1, FD&C blue # 2, FD&C red # 40, ferric oxide black, gelatin, lactose monohydrate, magnesium stearate, microcrystalline cellulose, n-butyl alcohol, propylene glycol, sda-3a alcohol, shellac, sodium lauryl sulfate, titanium dioxide and other inert ingredients.
The 15 mg capsule also contains: FD&C yellow # 6.
The 30 mg capsule also contains: D&C red # 28.